Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus
- PMID: 30592787
- PMCID: PMC6517235
- DOI: 10.1002/14651858.CD005061.pub3
Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus
Abstract
Background: Alpha-glucosidase inhibitors (AGI) reduce blood glucose levels and may thus prevent or delay type 2 diabetes mellitus (T2DM) and its associated complications in people at risk of developing of T2DM.
Objectives: To assess the effects of AGI in people with impaired glucose tolerance (IGT), impaired fasting blood glucose (IFG), moderately elevated glycosylated haemoglobin A1c (HbA1c) or any combination of these.
Search methods: We searched CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform, and the reference lists of systematic reviews, articles and health technology assessment reports. The date of the last search of all databases was December 2017.
Selection criteria: We included randomised controlled trials (RCTs), with a duration of one year or more, comparing AGI with any pharmacological glucose-lowering intervention, behaviour-changing intervention, placebo or no intervention in people with IFG, IGT, moderately elevated HbA1c or combinations of these.
Data collection and analysis: Two review authors read all abstracts and full-text articles or records, assessed quality and extracted outcome data independently. One review author extracted data, which were checked by a second review author. We resolved discrepancies by consensus or involvement of a third review author. For meta-analyses we used a random-effects model with assessment of risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, using 95% confidence intervals (CIs) for effect estimates. We assessed the overall quality of the evidence by using the GRADE instrument.
Main results: For this update of the Cochrane Review (first published 2006, Issue 4) we included 10 RCTs (11,814 participants), eight investigating acarbose and two investigating voglibose, that included people with IGT or people "at increased risk for diabetes". The trial duration ranged from one to six years. Most trials compared AGI with placebo (N = 4) or no intervention (N = 4).Acarbose reduced the incidence of T2DM compared to placebo: 670 out of 4014 people (16.7%) in the acarbose groups developed T2DM, compared to 812 out of 3994 people (20.3%) in the placebo groups (RR 0.82, 95% CI 0.75 to 0.89; P < 0.0001; 3 trials; 8008 participants; moderate-certainty evidence). One trial including participants with coronary heart disease and IGT contributed 64% of cases for this outcome. Acarbose reduced the risk of T2DM compared to no intervention: 7 out 75 people (9.3%) in the acarbose groups developed T2DM, compared to 18 out of 65 people (27.7%) in the no-intervention groups (RR 0.31, 95% CI 0.14 to 0.69; P = 0.004; 2 trials; 140 participants; very low-certainty evidence).Acarbose compared to placebo did not reduce or increase the risk of all-cause mortality (RR 0.98, 95% CI 0.82 to 1.18; P = 0.86; 3 trials; 8069 participants; very low-certainty evidence), cardiovascular mortality (RR 0.88; 95% CI 0.71 to 1.10; P = 0.26; 3 trials; 8069 participants; very low-certainty evidence), serious adverse events (RR 1.12, 95% CI 0.97 to 1.29; P = 0.13; 2 trials; 6625 participants; low-certainty evidence), non-fatal stroke (RR 0.50, 95% CI 0.09 to 2.74; P = 0.43; 1 trial; 1368 participants; very low-certainty evidence) or congestive heart failure (RR of 0.87; 95% CI 0.63 to 1.12; P = 0.40; 2 trials; 7890 participants; low-certainty evidence). Acarbose compared to placebo reduced non-fatal myocardial infarction: one out of 742 participants (0.1%) in the acarbose groups had a non-fatal myocardial infarction compared to 15 out of 744 participants (2%) in the placebo groups (RR 0.10, 95% CI 0.02 to 0.53; P = 0.007; 2 trials; 1486 participants; very low-certainty evidence). Acarbose treatment showed an increased risk of non-serious adverse events (mainly gastro-intestinal events), compared to placebo: 751 of 775 people (96.9%) in the acarbose groups experienced an event, compared to 723 of 775 people (93.3%) in the placebo groups (RR 1.04; 95% CI 1.01 to 1.06; P = 0.0008; 2 trials; 1550 participants). Acarbose compared to no intervention showed no advantage or disadvantage for any of these outcome measures (very low-certainty evidence).One trial each compared voglibose with placebo (1780 participants) or diet and exercise (870 participants). Voglibose compared to placebo reduced the incidence of T2DM: 50 out of 897 participants (5.6%) developed T2DM, compared to 106 out of 881 participants (12%) in the placebo group (RR 0.46, 95% CI 0.34 to 0.64; P < 0.0001; 1 trial; 1778 participants; low-certainty evidence). For all other reported outcome measures there were no clear differences between voglibose and comparator groups. One trial with 90 participants compared acarbose with diet and exercise and another trial with 98 participants reported data on acarbose versus metformin. There were no clear differences for any outcome measure between these two acarbose interventions and the associated comparator groups.None of the trials reported amputation of lower extremity, blindness or severe vision loss, end-stage renal disease, health-related quality of life, time to progression to T2DM, or socioeconomic effects.
Authors' conclusions: AGI may prevent or delay the development of T2DM in people with IGT. There is no firm evidence that AGI have a beneficial effect on cardiovascular mortality or cardiovascular events.
Conflict of interest statement
SM: none known PL: co‐author of a trial mentioned in the background and discussion (Van de Laar 2005). RA: co‐author of a trial mentioned in the background and discussion (Van de Laar 2005). WG: none known. FL: author of a trial mentioned in the background and discussion (Van de Laar 2005).
Figures

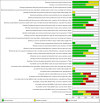
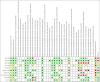
Update of
-
Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose.Cochrane Database Syst Rev. 2006 Oct 18;(4):CD005061. doi: 10.1002/14651858.CD005061.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2018 Dec 28;12:CD005061. doi: 10.1002/14651858.CD005061.pub3. PMID: 17054235 Updated.
References
References to studies included in this review
ABC 2017 {published and unpublished data}
-
- Asakura M, Kim J, Asanuma H, Hamasaki T, Tsukahara K, Higashino Y, et al. Does treatment of impaired glucose tolerance improve cardiovascular outcomes in patients with previous myocardial infarction?. Cardiovascular Drugs and Therapy 2017;31(4):401‐11. - PubMed
-
- NCT00212017. Assessment of an alpha‐glucosidase inhibitor to block cardiac events in patients with myocardial infarction and IGT (ABC Study). www.clinicaltrials.gov/ct2/show/NCT00212017 (accessed 10 July 2018).
ACE 2017 {published data only (unpublished sought but not used)}
-
- Diabetes Trials Unit, University of Oxford. Acarbose cardiovascular evaluation. www.dtu.ox.ac.uk/ACE (accessed 18 June 2018).
-
- Holman RR, Bethel MA, Chan JC, Chiasson J, Doran Z, Junbo G, et al. Rationale for and design of the Acarbose Cardiovascular Evaluation (ACE) trial. American Heart Journal 2014;168:23‐9. - PubMed
-
- Holman RR, Coleman RL, Chan JC, Chiasson J, Feng H, Junbo G, et al. for the ACE Study Group. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes & Endocrinology 2017;5:877‐86. - PubMed
-
- ISRCTN91899513. Acarbose Cardiovascular Evaluation trial (ACE). www.isrctn.com/ISRCTN91899513 (accessed 17 July 2018).
-
- NCT00829660. Acarbose Cardiovascular Evaluation Trial (ACE). www.clinicaltrials.gov/ct2/show/NCT00829660 (accessed 10 July 2018).
DAISI 2008 {published data only}
-
- ISRCTN33274262. Dutch acarbose intervention trial (DAISI). www.isrctn.com/ISRCTN33274262 (accessed 6 July 2018).
-
- Nijpels G, Boorsma W, Dekker JM, Kostense PJ, Bouter LM, Heine RJ. A study of the effects of acarbose on glucose metabolism in patients predisposed to developing diabetes: the Dutch Acarbose Intervention Study in persons with impaired glucose tolerance (DAISI). Diabetes/metabolism Research and Reviews 2008;24(8):611‐6. - PubMed
EDIT 1997 {published data only}
-
- Citroën HA, Tunbridge FKE, Holman RR. Possible prevention of type 2 diabetes with acarbose or metformin over three years. Diabetologia 2000;43(Suppl 1):A73.
-
- Diabetes Trials Unit, University of Oxford. Early diabetes intervention trial (protocol). www.dtu.ox.ac.uk/EDIT/protocol (accessed 6 July 2018).
-
- Holman RR, Blackwell L, Manley SE, Tucker L, Frighi V, Stratton IM. Results from the Early Diabetes Intervention Trial. Diabetes 2003;52(Suppl 1):A16.
-
- Holman RR, Blackwell L, Stratton IM, Manley SE, Tucker L, Frighi V. Six‐years results from the Early Diabetes Intervention Trial. Diabetic Medicine 2003;20(Suppl 2):15.
-
- Holman RR, North BV, Tunbridge FKE. Early Diabetes Intervention Trial. Diabetes 1997;46(Suppl 1):157A.
Fang 2004 {published data only}
-
- Fang YS, Li TY, Chen SY. Effect of medicine and non‐medicine intervention on the outcomes of patients with impaired glucose tolerance: 5‐year follow‐up. Zhongguo Linchuang Kangfu [Chinese Journal for Clinical Rehabilitation] 2004;8(30):6562‐3.
Kawamori 2009 {published data only (unpublished sought but not used)}
-
- Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Koku K, Voglibose Ph‐3 Study Group. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double‐blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373(9675):1607‐14. - PubMed
-
- UMIN000001109. A study in subjects with impaired glucose tolerance (IGT) to evaluate effects of AO‐128 on prevention of type 2 diabetes mellitus. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000001296 (accessed 17 July 2018).
Koyasu 2010 {published data only (unpublished sought but not used)}
-
- Koyasu M, Ishii H, Watarai M, Takemoto K, Inden Y, Takeshita K, et al. Impact of acarbose on carotid intima‐media thickness in patients with newly diagnosed impaired glucose tolerance or mild type 2 diabetes mellitus: a one‐year, prospective, randomized, open‐label, parallel‐group study in Japanese adults with established coronary artery disease. Clinical Therapeutics 2010;32(9):1610‐7. - PubMed
-
- UMIN000000544. Impact of acarbose therapy in abnormal glucose tolerance patients with coronary artery disease. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000000658 (accessed 17 July 2018).
STOP‐NIDDM 2002 {published and unpublished data}
-
- Anonymous. The "STOP NIDDM" program ‐ can diabetes in the aged be prevented? An international long‐term study revisited; does acarbose delay or prevent the manifestations of type II diabetes [STOP NIDDM Programm ‐ Kann man den Altersdiabetes verhindern? Eine internationale Langzeit‐Studie untersucht, ob Acarbose die Manifestation des Typ II‐Diabetes verzögert oder verhindert]. Deutsche Medizinische Wochenschrift 1997;122(38 Suppl):1‐4. - PubMed
-
- Anonymous. The STOP‐NIDDM trial study to prevent non insulin dependent diabetes mellitus [PowerPoint presentation]. www.stop‐niddm.com/study/slides/htm (accessed 1 August 2006; no longer available) 2003.
-
- Bridges CM. Acarbose for patients with hypertension and impaired glucose tolerance. JAMA 2003;290(23):3066‐7. - PubMed
-
- Chiasson JL. The potential use of acarbose in the prevention of type 2 diabetes and cardiovascular disease. European Heart Journal Supplements 2000;2(Supplement D):D35.
-
- Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP‐NIDDM Trial: an international study on the efficacy of an alpha‐glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: rationale, design, and preliminary screening data. Study to Prevent Non‐Insulin‐Dependent Diabetes Mellitus. Diabetes Care 1998;21(10):1720‐5. - PubMed
Wang 2000 {published data only}
-
- Wang H, Xu WH, Wang GY. An evalualion on efficacy of acarbose interfering trentment on IGT. Shanxi Clinical Medicine Journal 2000;9(2):116‐7.
References to studies excluded from this review
ABDOMEN study {published data only}
-
- NCT01167231. Prevention of postprandial hyperglycemia by acarbose may be a promising therapeutic strategy for reducing the increased risk for cardiovascular disease (ABDOMEN). www.clinicaltrials.gov/ct2/show/NCT01167231 (accessed 11 July 2018).
Aoki 2010 {published data only}
-
- UMIN000003170. Effect of miglitol and sitagliptin on incretin levels. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000003662 (accessed 11 July 2018).
EDIP {published data only}
-
- Perry RC, Shankar RR, Fineberg N, McGill J, Baron AD. HbA1c measurement improves the detection of type 2 diabetes in high‐risk individuals with nondiagnostic levels of fasting plasma glucose: the Early Diabetes Intervention Program (EDIP). Diabetes Care 2001;24(3):465‐71. - PubMed
-
- Shankar RR, Shankar SS, Brizendine E, Shen G, McGill J, Baron AD, et al. Acarbose in 'early' diabetes ‐ data from the Early Diabetes Intervention Program (EDIP). Diabetes 2005;54(Suppl 1):A132.
JEDIS study {published data only}
-
- UMIN000000681. A randomized comparative clinical study on suppression of progression from early diabetes, diet/exercise standard intervention vs. concurrent pharmacological standard intervention. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000000817 (accessed 11 July 2018).
Kataoka 2012 {published data only}
-
- Kataoka Y, Yasuda S, Miyamoto Y, Sase K, Kosuge M, Kimura K, et al. Effects of voglibose and nateglinide on glycemic status and coronary atherosclerosis in early‐stage diabetic patients. Circulation Journal 2012;76(3):712‐20. - PubMed
Mangiagli 2004 {published data only}
-
- Mangiagli A, Campisi S, Sanctis V, Nicoletti MC, Cardinale G, Galati MC, et al. Effects of acarbose in patients with beta‐thalassaemia major and abnormal glucose homeostasis. Pediatric Endocrinology Reviews 2004;2(Suppl 2):276‐8. - PubMed
Medizinische Klinik B study {published data only}
-
- 2004‐002579‐17. A double‐blind, randomised placebo controlled study of the effects of acarbose on endothelial function, hemostatic and fibrinolytic plasmatic factors in patients with stable coronary artery disease and either impaired glucose intolerance or newly diagnosed diabetes mellitus. www.clinicaltrialsregister.eu/ctr‐search/trial/2004‐002579‐17/DE (accessed 11 July 2018).
MM study {published data only}
-
- UMIN000010282. Three‐arm comparative study of miglitol and mitiglinide alone and in combination ‐efficacy and safety studies. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000012031 (accessed 11 July 2018).
Narita 2009 {published data only}
-
- UMIN000001671. The comparative study of the effects of miglitol and voglibose (alpha‐glucosidase inhibitors) on incretins secretion. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000002020 (accessed 11 July 2018).
NCT00417950 {published data only}
-
- NCT00417950. Additional glucose lowering and anti‐inflammatory effects by acarbose and alcohol in subjects with impaired glucose tolerance or mild type 2 diabetes. www.clinicaltrials.gov/ct2/show/NCT00417950 (accessed 11 July 2018).
Toyoda 2012 {published data only}
-
- UMIN000008692. Study of the combination use effect of DPP‐4 inhibitor and alpha‐glucosidase inhibitor. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000010217 (accessed 11 July 2018).
Watada 2012 {published data only}
-
- UMIN000008591. Linagliptin study of effects on PPG, postprandial blood glucose control. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000010093 (accessed 11 July 2018).
Yang 2001 {published data only}
-
- Yang W, Lin L, Qi J, Yu Z, Pei H, He G, et al. The preventive effect of acarbose and metformin on the progression to diabetes mellitus in the IGT population: a 3‐year multicenter prospective study [Chinese translation from Bayer website www.stop‐niddm.com, accessed 13 September 2004, no longer available]. Chinese Journal of Endocrinology and Metabolism 2001;17(3):131‐6.
References to studies awaiting assessment
NCT00221156 {published data only}
-
- NCT00221156. Acarbose and secondary prevention after coronary stenting. www.clinicaltrials.gov/ct2/show/NCT00221156 (accessed 18 December 2018).
Additional references
Abdul‐Ghani 2006
-
- Abdul‐Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55(5):1430‐5. [PUBMED: 16644701] - PubMed
ADA 2003
-
- American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26(suppl 1):s33‐s50. - PubMed
ADA 2010
ADA 2014
-
- American Diabetes Association. Strategies for effective screening, intervention and follow‐up. professional.diabetes.org/slidelibrary/prediabetes‐strategies‐effective‐... 2014 (accessed 7 December 2017).
ADA 2015
-
- American Diabetes Association. Standards of medical care in diabetes‐2015. Diabetes Care 2015;38(Suppl 1):S1‐93. [PUBMED: 24357209] - PubMed
Anderson 1991
-
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. American Heart Journal 1991;121(1 Pt 2):293‐8. - PubMed
Bell 2013
-
- Bell ML, McKenzie JE. Designing psycho‐oncology randomised trials and cluster randomised trials: variance components and intra‐cluster correlation of commonly used psychosocial measures. Psycho‐oncology 2013;22:1738‐47. - PubMed
Borenstein 2017a
-
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. - PubMed
Borenstein 2017b
-
- Borenstein M. Prediction intervals. www.meta‐analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
-
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. - PubMed
Caballero 2016
-
- Caballero AE. Long‐term studies of treatments for type 2 diabetes. Postgraduate Medicine 2016;129(3):352‐65. - PubMed
CDC 2015
-
- Centers for Disease Control and Prevention. 2014 National Diabetes Statistics Report. www.cdc.gov/diabetes/pdfs/data/2014‐report‐estimates‐of‐diabetes‐and‐its... (accessed 6 July 2018).
Chiasson 1996
-
- Chiasson JL, Josse RG, Leiter LA, Mihic M, Nathan DM, Palmason C, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care 1996;19(11):1190‐3. - PubMed
Colditz 1995
-
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Annals of Internal Medicine 1995;122(7):481‐6. - PubMed
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79‐85. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DeFronzo 1989
-
- DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism: Clinical and Experimental 1989;38(4):387‐95. [PUBMED: 2657323] - PubMed
Diabetes Prevention Program 2002
Diabetes Prevention Program FU 2009
Dunkley 2014
-
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta‐analysis. Diabetes Care 2014;37(4):922‐33. [PUBMED: 24652723] - PubMed
Finnish Diabetes Prevention Study Group 2001
-
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne‐Parikka P, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. - PubMed
Gosmanov 2014
-
- Gosmanov AR, Wan J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. American Journal of the Medical Sciences 2014;348(3):191‐4. [PUBMED: 24556928] - PubMed
GRADEproGDT 2015 [Computer program]
-
- McMaster University, 2015 (developed by Evidence Prime, Inc.). GRADEpro GDT. Version accessed 6 July 2018. Hamilton (ON): McMaster University, 2015 (developed by Evidence Prime, Inc.), 2015.
Hemmingsen 2016a
-
- Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012151] - DOI - PMC - PubMed
Hemmingsen 2016b
-
- Hemmingsen B, Krogh J, Metzendorf MI, Richter B. Sodium‐glucose cotransporter (SGLT) 2 inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012106.pub2] - DOI - PMC - PubMed
Hemmingsen 2017a
-
- Hemmingsen B, Gimenez‐Perez G, Mauricio D, Roqué i Figuls M, Metzendorf MI, et al. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD003054.pub4] - DOI - PMC - PubMed
Hemmingsen 2017b
-
- Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Dipeptidyl‐peptidase (DPP)‐4 inhibitors and glucagon‐like peptide (GLP)‐1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD012204.pub2] - DOI - PMC - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2009
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hopper 2011
-
- Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta‐analysis of randomised controlled clinical trials. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(6):813‐23. - PubMed
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. - PMC - PubMed
ICH 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. PA 19063‐2043. USA: Barnett International/PAREXEL, 1997.
IDF 2017
-
- International Diabetes Federation. IDF Diabetes Atlas. 8th Edition. Brussels, Belgium: International Diabetes Federation, 2017.
IEC 2009
InterAct Consortium 2013
Jenney 1993
-
- Jenney A, Proietto J, O'Dea K, Nankervis A, Traianedes K, D'Embden H. Low‐dose acarbose improves glycemic control in NIDDM patients without changes in insulin sensitivity. Diabetes Care 1993;16(2):499‐502. - PubMed
Jensen 2002
-
- Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta‐cell function is a major contributor to oral glucose tolerance in high‐risk relatives of four ethnic groups in the U.S. Diabetes 2002;51(7):2170‐8. [PUBMED: 12086947] - PubMed
Johnson 1996
-
- Johnson AB, Taylor R. Does suppression of postprandial blood glucose excursions by the alpha‐glucosidase inhibitor miglitol improve insulin sensitivity in diet‐treated type II diabetic patients?. Diabetes Care 2006;19(6):559‐63. - PubMed
Kaiser 2004
-
- Kaiser T, Sawicki PT. Acarbose for prevention of diabetes, hypertension and cardiovascular events? A critical analysis of the STOP‐NIDDM data. Diabetologia 2004;47(3):575‐80. - PubMed
Kirkham 2010
Kirkman 2006
-
- Kirkman MS, Shankar RR, Shankar S, Shen C, Brizendine E, Baron A, et al. Treating postprandial hyperglycemia does not appear to delay progression of early type 2 diabetes: the Early Diabetes Intervention Program. Diabetes Care 2006;29(9):2095‐101. - PubMed
Laakso 1999
-
- Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes 1999;48(5):937‐42. - PubMed
Liberati 2009
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. - PubMed
Matsumoto 1998
-
- Matsumoto K, Yano M, Miyake S, Ueki Y, Yamaguchi Y, Akazawa S, et al. Effects of voglibose on glycemic excursions, insulin secretion, and insulin sensitivity in non‐insulin‐treated NIDDM patients. Diabetes Care 1998;21(2):256‐60. - PubMed
Meader 2014
Meigs 2000
-
- Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 2000;49(12):2201‐7. - PubMed
Meneilly 2000
-
- Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, et al. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care 2000;23(8):1162‐7. - PubMed
Morris 2013
-
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0‐6.4% and other prediabetes definitions to type 2 diabetes: a meta‐analysis. Diabetologia 2013;56(7):1489‐93. [PUBMED: 23584433] - PubMed
Nguyen 2011
Pan 2003
-
- Pan CY, Gao Y, Chen JW, Luo BY, Fu ZZ, Lu JM, et al. Efficacy of acarbose in Chinese subjects with impaired glucose tolerance. Diabetes Research and Clinical Practice 2003;61:183–90. - PubMed
Quilici 2005
-
- Quilici S, Chancellor J, Maclaine G, McGuire A, Andersson D, Chiasson JL. Cost‐effectiveness of acarbose for the management of impaired glucose tolerance in Sweden. International Journal of Clinical Practice 2005;59(10):1143‐52. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. - PubMed
Sawicki 2004
-
- Sawicki PT, Kaiser T. Response to Chiasson et al.: acarbose for the prevention of type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP‐NIDDM Trial data. Diabetologia 2004;47(6):976‐7. - PubMed
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Selvin 2011
Shinozaki 1996
-
- Shinozaki K, Suzuki M, Ikebuchi M, Hirose J, Hara Y, Harano Y. Improvement of insulin sensitivity and dyslipidemia with a new alpha‐glucosidase inhibitor, voglibose, in nondiabetic hyperinsulinemic subjects. Metabolism: Clinical and Experimental 1996;45(6):731‐7. - PubMed
Stern 2002
-
- Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test?. Annals of Internal Medicine 2002;136(8):575‐81. - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Stevens 2015
-
- Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Woods HB, et al. Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta‐analysis of lifestyle, pharmacological and surgical interventions. Diabetes Research and Clinical Practice 2015;107(3):320‐31. - PubMed
Stumvoll 2005
-
- Stumvoll M, Goldstein BJ, Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005;365(9467):1333‐46. - PubMed
Tabak 2012
Turnbull 2009
-
- Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52(11):2288‐98. - PubMed
Van de Laar 2005
WHO 1985
-
- World Health Organisation. Technical Report Series No. 727. Report of a WHO Study Group. WHO Expert Committee on Diabetes Mellitus 1985.
WHO 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. - PubMed
WHO/IDF 2006
-
- World Health Organization/International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20di... 2006 (accessed 6 July 2018).
Wong 2006a
Wong 2006b
Wood 2008
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

