Functional analysis of the role of CcpA in Lactobacillus plantarum grown on fructooligosaccharides or glucose: a transcriptomic perspective
- PMID: 30593274
- PMCID: PMC6309078
- DOI: 10.1186/s12934-018-1050-4
Functional analysis of the role of CcpA in Lactobacillus plantarum grown on fructooligosaccharides or glucose: a transcriptomic perspective
Abstract
Background: The catabolite control protein A (CcpA) is a master regulator of many important cellular processes in Gram-positive bacteria. In Lactobacillus plantarum, CcpA directly or indirectly controls the transcription of a large number of genes that are involved in carbohydrate metabolism, aerobic and anaerobic growth, stress response and metabolite production, but its role in response to different carbon sources remains unclear.
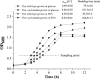
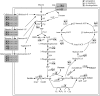
Results: Here a combined transcriptomic and physiological approach was used to survey the global alterations that occurred during the logarithmic growth phase of wild-type and ccpA mutant strains of L. plantarum ST-III using fructooligosaccharides (FOS) or glucose as the sole carbon source. The inactivation of ccpA significantly affected the growth and production of metabolites under both carbon sources. About 15% of the total genes were significantly altered between wild-type and ccpA strains grown on glucose and the value is deceased to 12% when these two strains were compared on FOS, while only 7% were obviously changed due to the loss of CcpA when comparing strains grown on glucose and FOS. Although most of the differentially expressed genes mediated by CcpA are glucose dependent, FOS can also induce carbon catabolite repression (CCR) through the CcpA pathway. Moreover, the inactivation of ccpA led to a transformation from homolactic fermentation to mixed fermentation under aerobic conditions. CcpA can control genes directly by binding in the regulatory region of the target genes (mixed fermentation), indirectly through local regulators (fatty acid biosynthesis), or have a double effect via direct and indirect regulation (FOS metabolism).
Conclusion: Overall, our results show that CcpA plays a central role in response to carbon source and availability of L. plantarum and provide new insights into the complex and extended regulatory network of lactic acid bacteria.
Keywords: Carbohydrate metabolism; Catabolite control protein A; Fatty acid biosynthesis; Lactobacillus plantarum; Mixed fermentation; Transcriptome.
Figures

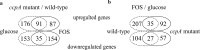
Similar articles
-
The local transcriptional regulators SacR1 and SacR2 act as repressors of fructooligosaccharides metabolism in Lactobacillus plantarum.Microb Cell Fact. 2020 Aug 10;19(1):161. doi: 10.1186/s12934-020-01403-3. Microb Cell Fact. 2020. PMID: 32778113 Free PMC article.
-
Comparative Transcriptional Analysis of Lactobacillus plantarum and Its ccpA-Knockout Mutant Under Galactooligosaccharides and Glucose Conditions.Front Microbiol. 2019 Jul 9;10:1584. doi: 10.3389/fmicb.2019.01584. eCollection 2019. Front Microbiol. 2019. PMID: 31338086 Free PMC article.
-
Metabolism of Fructooligosaccharides in Lactobacillus plantarum ST-III via Differential Gene Transcription and Alteration of Cell Membrane Fluidity.Appl Environ Microbiol. 2015 Nov;81(22):7697-707. doi: 10.1128/AEM.02426-15. Epub 2015 Aug 28. Appl Environ Microbiol. 2015. PMID: 26319882 Free PMC article.
-
Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization.FEMS Microbiol Lett. 2002 Apr 9;209(2):141-8. doi: 10.1111/j.1574-6968.2002.tb11123.x. FEMS Microbiol Lett. 2002. PMID: 12007797 Review.
-
Carbon catabolite repression by the catabolite control protein CcpA in Staphylococcus xylosus.J Mol Microbiol Biotechnol. 2002 May;4(3):309-14. J Mol Microbiol Biotechnol. 2002. PMID: 11931563 Review.
Cited by
-
Reconstructing the transcriptional regulatory network of probiotic L. reuteri is enabled by transcriptomics and machine learning.mSystems. 2024 Mar 19;9(3):e0125723. doi: 10.1128/msystems.01257-23. Epub 2024 Feb 13. mSystems. 2024. PMID: 38349131 Free PMC article.
-
Recent progress on n-butanol production by lactic acid bacteria.World J Microbiol Biotechnol. 2021 Oct 26;37(12):205. doi: 10.1007/s11274-021-03173-5. World J Microbiol Biotechnol. 2021. PMID: 34698975 Review.
-
Genome-wide transcriptomics revealed carbon source-mediated gamma-aminobutyric acid (GABA) production in a probiotic, Lactiplantibacillus pentosus 9D3.Heliyon. 2025 Jan 10;11(2):e41879. doi: 10.1016/j.heliyon.2025.e41879. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39897778 Free PMC article.
-
Transcriptome, Phenotypic, and Virulence Analysis of Streptococcus sanguinis SK36 Wild Type and Its CcpA-Null Derivative (ΔCcpA).Front Cell Infect Microbiol. 2019 Dec 4;9:411. doi: 10.3389/fcimb.2019.00411. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31867286 Free PMC article.
-
Improving rhamnolipids production using fermentation-foam fractionation coupling system: cell immobilization and waste frying oil emulsion.Bioprocess Biosyst Eng. 2023 Aug;46(8):1175-1194. doi: 10.1007/s00449-023-02890-5. Epub 2023 Jun 20. Bioprocess Biosyst Eng. 2023. PMID: 37338581
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

