Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy
- PMID: 30593276
- PMCID: PMC6309092
- DOI: 10.1186/s12943-018-0926-6
Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy
Abstract
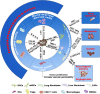
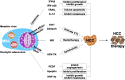
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, with high morbidity, relapse and mortality rates. Multipotent mesenchymal stromal cells (MSCs) can be recruited to and become integral components of the HCC microenvironment and can influence tumor progression. This review discusses MSC migration to liver fibrosis and the HCC microenvironment, MSC involvement in HCC initiation and progression and the widespread application of MSCs in HCC-targeted therapy, thus clarifying the critical roles of MSCs in HCC.
Keywords: Carcinogenesis; Chemotaxis; Hepatocellular carcinoma (HCC); Mesenchymal stromal cells (MSCs); Neoplasm metastasis; Tumor-targeted therapy.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

