Bladder cancer extracellular vesicles drive tumorigenesis by inducing the unfolded protein response in endoplasmic reticulum of nonmalignant cells
- PMID: 30593508
- PMCID: PMC6398136
- DOI: 10.1074/jbc.RA118.006682
Bladder cancer extracellular vesicles drive tumorigenesis by inducing the unfolded protein response in endoplasmic reticulum of nonmalignant cells
Abstract
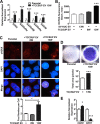
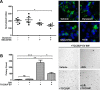
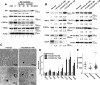
The field cancerization effect has been proposed to explain bladder cancer's multifocal and recurrent nature, yet the mechanisms of this effect remain unknown. In this work, using cell biology, flow cytometry, and qPCR analyses, along with a xenograft mouse tumor model, we show that chronic exposure to tumor-derived extracellular vesicles (TEVs) results in the neoplastic transformation of nonmalignant human SV-HUC urothelial cells. Inhibition of EV uptake prevented this transformation. Transformed cells not only possessed several oncogenic properties, such as increased genome instability, loss of cell-cell contact inhibition, and invasiveness, but also displayed altered morphology and cell structures, such as an enlarged cytoplasm with disrupted endoplasmic reticulum (ER) alignment and the accumulation of smaller mitochondria. Exposure of SV-HUC cells to TEVs provoked the unfolded protein response in the endoplasmic reticulum (UPRER). Prolonged induction of UPRER signaling activated the survival branch of the UPRER pathway, in which cells had elevated expression of inositol-requiring enzyme 1 (IRE1), NF-κB, and the inflammatory cytokine leptin, and incurred loss of the pro-apoptotic protein C/EBP homologous protein (CHOP). More importantly, inhibition of ER stress by docosahexaenoic acid prevented TEV-induced transformation. We propose that TEVs promote malignant transformation of predisposed cells by inhibiting pro-apoptotic signals and activating tumor-promoting ER stress-induced unfolded protein response and inflammation. This study provides detailed insight into the mechanisms underlying the bladder cancer field effect and tumor recurrence.
Keywords: bladder cancer; cell stress; cytokine; endoplasmic reticulum stress (ER stress); exosome (vesicle); extracellular vesicles; filed cancerization; inflammation; unfolded protein response (UPR); urothelial carcinoma.
© 2019 Wu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Comment in
-
Re: Bladder Cancer Extracellular Vesicles Drive Tumorigenesis by Inducing the Unfolded Protein Response in Endoplasmic Reticulum of Nonmalignant Cells.J Urol. 2019 Dec;202(6):1100-1101. doi: 10.1097/01.JU.0000585152.03649.98. Epub 2019 Sep 12. J Urol. 2019. PMID: 31512971 No abstract available.
References
-
- Abdollah F., Gandaglia G., Thuret R., Schmitges J., Tian Z., Jeldres C., Passoni N. M., Briganti A., Shariat S. F., Perrotte P., Montorsi F., Karakiewicz P. I., and Sun M. (2013) Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 37, 219–225 10.1016/j.canep.2013.02.002 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

