Adapting clinical trial design to maintain meaningful outcomes during a multicenter asthma trial in the precision medicine era
- PMID: 30593883
- PMCID: PMC6425934
- DOI: 10.1016/j.cct.2018.12.012
Adapting clinical trial design to maintain meaningful outcomes during a multicenter asthma trial in the precision medicine era
Abstract
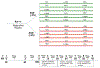
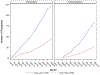
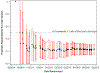
Precision medicine is expected to impact the care of people with asthma, given its high disease prevalence, heterogeneity of pathophysiologic mechanisms, and consequent clinical phenotypes. A novel phenotype-stratified clinical trial conducted by the NHLBI AsthmaNet Consortium, titled Steroids in Eosinophil Negative Asthma (SIENA), was a randomized, multicenter, clinical trial that prospectively stratified individuals according to their baseline level of sputum inflammation during a screening period. Two phenotypic strata were assigned based on an a priori defined extent of sputum eosinophilia (Eos Low versus Eos High). This article describes: the scientific premise for the trial design, including assumptions used for power calculations; modifications to the analysis plan implemented after the trial started due to a higher than expected prevalence of one phenotypic stratum which impacted the ability to accrue sufficient subjects within the planned budget and study period; investigator alternatives to address the strata imbalance weighing scientific impact and study feasibility; and the final modified SIENA study design and analysis plan. SIENA was successfully completed in a manner that maintained meaningful outcomes. We conclude with recommendations for incorporation of pre-specified contingency plans into phenotype-directed protocols, to address the potential for differences in observed compared to estimated prevalence of different phenotypes in a study population. These approaches can be applied to precision medicine trials for the future.
Trial registration: ClinicalTrials.gov NCT02066298.
Keywords: Biomarker-stratified; Induced sputum eosinophilia; Multicenter clinical trial; Participant recruitment; Phenotype-stratified; Precision medicine; Study design.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
References
-
- Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011;127:355–60. - PubMed
-
- Martin RJ, Szefler SJ, Chinchilli VM, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med 2002;165:1377–83. - PubMed
-
- Deykin A, Lazarus SC, Fahy JV, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol 2005;115:720–7. - PubMed
-
- Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet 1999;353:2213–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL098107/HL/NHLBI NIH HHS/United States
- U10 HL098075/HL/NHLBI NIH HHS/United States
- U10 HL098096/HL/NHLBI NIH HHS/United States
- U10 HL098102/HL/NHLBI NIH HHS/United States
- U10 HL098115/HL/NHLBI NIH HHS/United States
- U10 HL098177/HL/NHLBI NIH HHS/United States
- U10 HL098103/HL/NHLBI NIH HHS/United States
- P01 AI148104/AI/NIAID NIH HHS/United States
- U10 HL098098/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- U10 HL098090/HL/NHLBI NIH HHS/United States
- U10 HL098112/HL/NHLBI NIH HHS/United States

