Oncologic Outcomes in Metastatic Colorectal Cancer with Regorafenib with FOLFIRI as a Third- or Fourth-Line Setting
- PMID: 30594039
- PMCID: PMC6307535
- DOI: 10.1016/j.tranon.2018.12.003
Oncologic Outcomes in Metastatic Colorectal Cancer with Regorafenib with FOLFIRI as a Third- or Fourth-Line Setting
Abstract
Background: To evaluate the efficacy and toxicities of regorafenib plus irinotecan, dose-escalated on the basis of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping, in previously heavily treated metastatic colorectal cancer (mCRC) and the prognostic values of EGFR expression, KRAS mutations, and tumor sidedness.
Methods: Forty-one patients with mCRC with disease progression after treatment with fluoropyrimidines, oxaliplatin, irinotecan, anti-VEGF, and anti-EGFR MoAbs were subjected to UGT1A1 genotyping and received regorafenib combined with FOLFIRI with dose-escalated irinotecan.
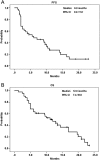
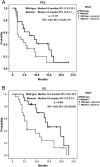
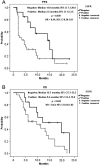
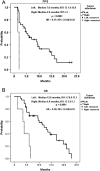
Results: The median follow-up period was 10.0 months (1.3-23.5 months). The overall disease control rate was 58.5%, whereas the median progression-free survival (PFS) and overall survival (OS) were 6.0 months and 12.0 months, respectively. KRAS mutations were significantly associated with positive EGFR expression (P = .026). KRAS mutations significantly correlated with a shorter OS than KRAS wild-type (6.0 vs. 14.4 months, P = .014) but had no significant association with PFS. Positive EGFR expression had an inverse correlation with PFS (2.5 vs. 14.0 months, P = .039) and OS (9.6 vs. 19.7 months, P = .044). Moreover, left-sided tumors associated with superior PFS (2.0 vs. 7.0 months, P < .0001) and OS (4.0 vs. 13.0 months, P < .0001), and tumor sidedness was an independent prognostic factor by the multivariate analysis.
Conclusion: Regorafenib and FOLFIRI concomitant therapy with dose-escalated irinotecan seemed to be potentially practicable with satisfactory oncological results. KRAS mutations and EGFR expression might be predictors of poor oncological outcomes; however, left-sided mCRCs would be more beneficial for concomitant regorafenib and FOLFIRI therapy.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Regorafenib plus FOLFIRI with irinotecan dose escalated according to uridine diphosphate glucuronosyltransferase 1A1genotyping in previous treated metastatic colorectal cancer patients:study protocol for a randomized controlled trial.Trials. 2019 Dec 19;20(1):751. doi: 10.1186/s13063-019-3917-z. Trials. 2019. PMID: 31856912 Free PMC article.
-
Regorafenib Plus FOLFIRI With Irinotecan Dose Escalated According to Uridine Diphosphate Glucuronosyltransferase 1A1 Genotyping in Patients With Metastatic Colorectal Cancer.Oncol Res. 2017 May 24;25(5):673-679. doi: 10.3727/97818823455816X14786040691928. Epub 2016 Nov 17. Oncol Res. 2017. PMID: 27938508 Free PMC article.
-
Determination of the UGT1A1 polymorphism as guidance for irinotecan dose escalation in metastatic colorectal cancer treated with first-line bevacizumab and FOLFIRI (PURE FIST).Eur J Cancer. 2020 Oct;138:19-29. doi: 10.1016/j.ejca.2020.05.031. Epub 2020 Aug 20. Eur J Cancer. 2020. PMID: 32829105 Clinical Trial.
-
Chemotherapy of metastatic colorectal cancer.Prescrire Int. 2010 Oct;19(109):219-24. Prescrire Int. 2010. PMID: 21180382 Review.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
Cited by
-
Personalized medicine in colorectal cancer.Gastroenterol Hepatol Bed Bench. 2020 Winter;13(Suppl1):S18-S28. Gastroenterol Hepatol Bed Bench. 2020. PMID: 33585000 Free PMC article. Review.
-
Regorafenib in Refractory Metastatic Colorectal Cancer: A Multi-Center Retrospective Study.Front Oncol. 2022 Mar 30;12:838870. doi: 10.3389/fonc.2022.838870. eCollection 2022. Front Oncol. 2022. PMID: 35433423 Free PMC article.
-
Durable responses to TAS-102 plus bevacizumab and TACE in salvage-line treatment of KRAS-mutated and MSS metastatic colorectal cancer: a case report.Am J Transl Res. 2023 Jul 15;15(7):4805-4812. eCollection 2023. Am J Transl Res. 2023. PMID: 37560229 Free PMC article.
-
Regorafenib plus FOLFIRI with irinotecan dose escalated according to uridine diphosphate glucuronosyltransferase 1A1genotyping in previous treated metastatic colorectal cancer patients:study protocol for a randomized controlled trial.Trials. 2019 Dec 19;20(1):751. doi: 10.1186/s13063-019-3917-z. Trials. 2019. PMID: 31856912 Free PMC article.
-
Case report: Efficacy and safety of regorafenib plus fluorouracil combination therapy in the treatment of refractory metastatic colorectal cancer.Front Oncol. 2022 Dec 15;12:992455. doi: 10.3389/fonc.2022.992455. eCollection 2022. Front Oncol. 2022. PMID: 36620581 Free PMC article.
References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. - PubMed
-
- Arnold D, Stein A. New developments in the second-line treatment of metastatic colorectal cancer: potential place in therapy. Drugs. 2013;73:883–891. - PubMed
-
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. - PubMed
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. - PubMed
-
- Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

