Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21-5p/Smad7 Pathway
- PMID: 30594070
- PMCID: PMC6307349
- DOI: 10.1016/j.omtn.2018.11.006
Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21-5p/Smad7 Pathway
Abstract
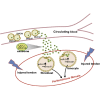
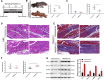
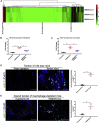
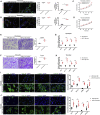
Following tendon injury, the development of fibrotic healing response impairs tendon function and restricts tendon motion. Peritendinous tissue fibrosis poses a major clinical problem in hand surgery. Communication between macrophages and tendon cells has a critical role in regulating the tendon-healing process. Yet, the mechanisms employed by macrophages to control peritendinous fibrosis are not fully understood. Here we analyze the role of macrophages in tendon adhesion in mice by pharmacologically depleting them. Such macrophage-depleted mice have less peritendinous fibrosis formation around the injured tendon compared with wild-type littermates. Macrophage-depleted mice restart fibrotic tendon healing by treatment with bone marrow macrophage-derived exosomes. We show that bone marrow macrophages secrete exosomal miR-21-5p that directly targets Smad7, leading to the activation of fibrogenesis in tendon cells. These results demonstrate that intercellular crosstalk between bone marrow macrophages and tendon cells is mediated by macrophage-derived miR-21-5p-containing exosomes that control the fibrotic healing response, providing potential targets for the prevention and treatment of tendon adhesion.
Keywords: Smad7; bone marrow macrophage; exosome; miR-21-5p; peritendinous adhesion.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Zhou Y.L., Yang Q.Q., Yan Y.Y., Zhu C., Zhang L., Tang J.B. Localized delivery of miRNAs targets cyclooxygenases and reduces flexor tendon adhesions. Acta Biomater. 2018;70:237–248. - PubMed
-
- Sharma P., Maffulli N. Tendon injury and tendinopathy: healing and repair. J. Bone Joint Surg. Am. 2005;87:187–202. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

