Clinical phenotypes and biologic treatment use in juvenile dermatomyositis-associated calcinosis
- PMID: 30594206
- PMCID: PMC6311016
- DOI: 10.1186/s12969-018-0299-9
Clinical phenotypes and biologic treatment use in juvenile dermatomyositis-associated calcinosis
Abstract
Background: Few risk factors have been identified for the development of calcinosis among patients with Juvenile Dermatomyositis, and currently no clinical phenotype has been associated with its development. We analyzed a large database of patients to further elucidate any relationships among patients with and without calcinosis.
Method: The CARRA legacy registry recruited pediatric rheumatology patients from 55 centers across North America from 2010 through 2014, including over 650 subjects with Juvenile Dermatomyositis. We compared the demographic characteristics, clinical disease features and treatment histories of those with and without calcinosis using univariate and multivariate logistic regression.
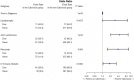
Results: Of the 631 patients included in the analysis, 84 (13%) had a current or prior history of calcinosis. These patients were statistically more likely to have longer durations of disease prior to diagnosis and treatment, have lipodystrophy and joint contractures, and to have received intravenous immune globulin or rituximab as treatments.
Conclusions: Calcinosis is found more often in patients with prolonged active disease, severe disease, and certain clinical features such as lipodystrophy and joint contractures. When these factors are combined with other known associations and predictors, groups of at-risk patients can be more effectively identified, treated and studied to improve overall outcomes.
Keywords: Biologics; Calcinosis; Juvenile dermatomyositis; Pediatric rheumatology.
Conflict of interest statement
Ethics approval and consent to participate
This study was exempt from review by the Washington University Institutional Review Board by not constituting human subject research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, Bowyer S, Dyer A, et al. US incidence of juvenile dermatomyositis, 1995-1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases registry. Arthritis Rheum. 2003;49(3):300–305. doi: 10.1002/art.11122. - DOI - PubMed
-
- Sullivan DB, Cassidy JT, Petty RE. Dermatomyositis in the pediatric patient. Arthritis Rheum. 1977;20(2 Suppl):327–331. - PubMed
-
- Jacobs JC. Methotrexate and azathioprine treatment of childhood dermatomyositis. Pediatrics. 1977;59(2):212–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases