SUMOylation regulates LKB1 localization and its oncogenic activity in liver cancer
- PMID: 30594553
- PMCID: PMC6412020
- DOI: 10.1016/j.ebiom.2018.12.031
SUMOylation regulates LKB1 localization and its oncogenic activity in liver cancer
Abstract
Background: Even though liver kinase B1 (LKB1) is usually described as a tumor suppressor in a wide variety of tissues, it has been shown that LKB1 aberrant expression is associated with bad prognosis in Hepatocellular Carcinoma (HCC).
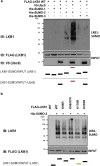
Methods: Herein we have overexpressed LKB1 in human hepatoma cells and by using histidine pull-down assay we have investigated the role of the hypoxia-related post-translational modification of Small Ubiquitin-related Modifier (SUMO)ylation in the regulation of LKB1 oncogenic role. Molecular modelling between LKB1 and its interactors, involved in regulation of LKB1 nucleocytoplasmic shuttling and LKB1 activity, was performed. Finally, high affinity SUMO binding entities-based technology were used to validate our findings in a pre-clinical mouse model and in clinical HCC.
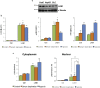
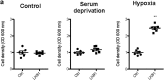
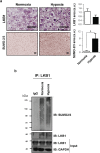
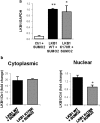
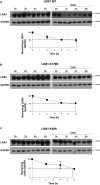
Findings: We found that in human hepatoma cells under hypoxic stress, LKB1 overexpression increases cell viability and aggressiveness in association with changes in LKB1 cellular localization. Moreover, by using site-directed mutagenesis, we have shown that LKB1 is SUMOylated by SUMO-2 at Lys178 hampering LKB1 nucleocytoplasmic shuttling and fueling hepatoma cell growth. Molecular modelling of SUMO modified LKB1 further confirmed steric impedance between SUMOylated LKB1 and the STe20-Related ADaptor cofactor (STRADα), involved in LKB1 export from the nucleus. Finally, we provide evidence that endogenous LKB1 is modified by SUMO in pre-clinical mouse models of HCC and clinical HCC, where LKB1 SUMOylation is higher in fast growing tumors.
Interpretation: Overall, SUMO-2 modification of LKB1 at Lys178 mediates LKB1 cellular localization and its oncogenic role in liver cancer. FUND: This work was supported by grants from NIH (US Department of Health and Human services)-R01AR001576-11A1 (J.M.M and M.L.M-C.), Gobierno Vasco-Departamento de Salud 2013111114 (to M.L.M.-C), ELKARTEK 2016, Departamento de Industria del Gobierno Vasco (to M.L.M.-C), MINECO: SAF2017-87301-R and SAF2014-52097-R integrado en el Plan Estatal de Investigación Cientifica y Técnica y Innovación 2013-2016 cofinanciado con Fondos FEDER (to M.L.M.-C and J.M.M., respectively), BFU2015-71017/BMC MINECO/FEDER, EU (to A.D.Q. and I.D.M.), BIOEF (Basque Foundation for Innovation and Health Research): EITB Maratoia BIO15/CA/014; Instituto de Salud Carlos III:PIE14/00031, integrado en el Plan Estatal de Investigación Cientifica y Técnica y Innovacion 2013-2016 cofinanciado con Fondos FEDER (to M.L.M.-C and J.M.M), Asociación Española contra el Cáncer (T.C.D, P·F-T and M.L.M-C), Daniel Alagille award from EASL (to T.C.D), Fundación Científica de la Asociación Española Contra el Cancer (AECC Scientific Foundation) Rare Tumor Calls 2017 (to M.L.M and M.A), La Caixa Foundation Program (to M.L.M), Programma di Ricerca Regione-Università 2007-2009 and 2011-2012, Regione Emilia-Romagna (to E.V.), Ramón Areces Foundation and the Andalusian Government (BIO-198) (A.D.Q. and I.D.M.), ayudas para apoyar grupos de investigación del sistema Universitario Vasco IT971-16 (P.A.), MINECO:SAF2015-64352-R (P.A.), Institut National du Cancer, FRANCE, INCa grant PLBIO16-251 (M.S.R.), MINECO - BFU2016-76872-R to (E.B.). Work produced with the support of a 2017 Leonardo Grant for Researchers and Cultural Creators, BBVA Foundation (M.V-R). Finally, Ciberehd_ISCIII_MINECO is funded by the Instituto de Salud Carlos III. We thank MINECO for the Severo Ochoa Excellence Accreditation to CIC bioGUNE (SEV-2016-0644). Funding sources had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Keywords: HCC; LKB1; SIRT1; STRADα; SUMO.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
SUMO-Binding Entities (SUBEs) as Tools for the Enrichment, Isolation, Identification, and Characterization of the SUMO Proteome in Liver Cancer.J Vis Exp. 2019 Nov 1;(153). doi: 10.3791/60098. J Vis Exp. 2019. PMID: 31736480
-
Activation of LKB1-Akt pathway independent of phosphoinositide 3-kinase plays a critical role in the proliferation of hepatocellular carcinoma from nonalcoholic steatohepatitis.Hepatology. 2010 Nov;52(5):1621-31. doi: 10.1002/hep.23860. Hepatology. 2010. PMID: 20815019 Free PMC article.
-
Upregulation of liver kinase B1 predicts poor prognosis in hepatocellular carcinoma.Int J Oncol. 2018 Nov;53(5):1913-1926. doi: 10.3892/ijo.2018.4556. Epub 2018 Sep 7. Int J Oncol. 2018. PMID: 30226588 Free PMC article.
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
Sumoylation in liver disease.Clin Chim Acta. 2020 Nov;510:347-353. doi: 10.1016/j.cca.2020.07.044. Epub 2020 Jul 23. Clin Chim Acta. 2020. PMID: 32710938 Review.
Cited by
-
Bioinformatics-based screening of key genes for transformation of liver cirrhosis to hepatocellular carcinoma.J Transl Med. 2020 Jan 30;18(1):40. doi: 10.1186/s12967-020-02229-8. J Transl Med. 2020. PMID: 32000807 Free PMC article.
-
Sphingolipids in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma: Ceramide Turnover.Int J Mol Sci. 2019 Dec 19;21(1):40. doi: 10.3390/ijms21010040. Int J Mol Sci. 2019. PMID: 31861664 Free PMC article. Review.
-
SUMOylation inhibitors activate anti-tumor immunity by reshaping the immune microenvironment in a preclinical model of hepatocellular carcinoma.Cell Oncol (Dordr). 2024 Apr;47(2):513-532. doi: 10.1007/s13402-023-00880-z. Epub 2023 Dec 6. Cell Oncol (Dordr). 2024. PMID: 38055116
-
Epigenetic remodelling in human hepatocellular carcinoma.J Exp Clin Cancer Res. 2022 Mar 24;41(1):107. doi: 10.1186/s13046-022-02297-2. J Exp Clin Cancer Res. 2022. PMID: 35331312 Free PMC article. Review.
-
Computational Characterizing Necroptosis Reveals Implications for Immune Infiltration and Immunotherapy of Hepatocellular Carcinoma.Front Oncol. 2022 Jul 7;12:933210. doi: 10.3389/fonc.2022.933210. eCollection 2022. Front Oncol. 2022. PMID: 35875102 Free PMC article.
References
-
- Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391(6663):184–187. - PubMed
-
- Woods A., Johnstone S.R., Dickerson K., Leiper F.C., Fryer L.G., Neumann D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–2008. - PubMed
-
- Lee M., Hwang J.T., Lee H.J., Jung S.N., Kang I., Chi S.G. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278(41):39653–39661. - PubMed
-
- Bolster D.R., Crozier S.J., Kimball S.R., Jefferson L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277(27):23977–23980. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

