5-Aminothiophene-2,4-dicarboxamide analogues as hepatitis B virus capsid assembly effectors
- PMID: 30594676
- PMCID: PMC6362850
- DOI: 10.1016/j.ejmech.2018.12.047
5-Aminothiophene-2,4-dicarboxamide analogues as hepatitis B virus capsid assembly effectors
Abstract
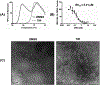
Chronic hepatitis B virus (HBV) infection represents a major health threat. Current FDA-approved drugs do not cure HBV. Targeting HBV core protein (Cp) provides an attractive approach toward HBV inhibition and possibly infection cure. We have previously identified and characterized a 5-amino-3-methylthiophene-2,4-dicarboxamide (ATDC) compound as a structurally novel hit for capsid assembly effectors (CAEs). We report herein hit validation through studies on absorption, distribution, metabolism and excretion (ADME) properties and pharmacokinetics (PK), and hit optimization via analogue synthesis aiming to probe the structure-activity relationship (SAR) and structure-property relationship (SPR). In the end, these medicinal chemistry efforts led to the identification of multiple analogues strongly binding to Cp, potently inhibiting HBV replication in nanomolar range without cytotoxicity, and exhibiting good oral bioavailability (F). Two of our analogues, 19o (EC50 = 0.11 μM, CC50 > 100 μM, F = 25%) and 19k (EC50 = 0.31 μM, CC50 > 100 μM, F = 46%), displayed overall lead profiles superior to reported CAEs 7-10 used in our studies.
Copyright © 2018 Elsevier Masson SAS. All rights reserved.
Figures

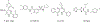
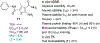

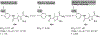

Similar articles
-
Optimization and Synthesis of Pyridazinone Derivatives as Novel Inhibitors of Hepatitis B Virus by Inducing Genome-free Capsid Formation.ACS Infect Dis. 2017 Mar 10;3(3):199-205. doi: 10.1021/acsinfecdis.6b00159. Epub 2016 Dec 29. ACS Infect Dis. 2017. PMID: 27989113
-
Preclinical Characterization of NVR 3-778, a First-in-Class Capsid Assembly Modulator against Hepatitis B Virus.Antimicrob Agents Chemother. 2018 Dec 21;63(1):e01734-18. doi: 10.1128/AAC.01734-18. Print 2019 Jan. Antimicrob Agents Chemother. 2018. PMID: 30373799 Free PMC article.
-
Discovery and Mechanistic Study of Benzamide Derivatives That Modulate Hepatitis B Virus Capsid Assembly.J Virol. 2017 Jul 27;91(16):e00519-17. doi: 10.1128/JVI.00519-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28566379 Free PMC article.
-
Targeting the multifunctional HBV core protein as a potential cure for chronic hepatitis B.Antiviral Res. 2020 Oct;182:104917. doi: 10.1016/j.antiviral.2020.104917. Epub 2020 Aug 17. Antiviral Res. 2020. PMID: 32818519 Free PMC article. Review.
-
Review of small synthetic molecules targeting HBV capsid assembly.Med Chem. 2015;11(8):710-6. doi: 10.2174/157340641108151029111243. Med Chem. 2015. PMID: 25985859 Review.
Cited by
-
Novel Hepatitis B Virus Capsid Assembly Modulator Induces Potent Antiviral Responses In Vitro and in Humanized Mice.Antimicrob Agents Chemother. 2020 Jan 27;64(2):e01701-19. doi: 10.1128/AAC.01701-19. Print 2020 Jan 27. Antimicrob Agents Chemother. 2020. PMID: 31712213 Free PMC article.
-
New Synthetic Methodology for Drug-like Molecules.Molecules. 2023 Jul 26;28(15):5632. doi: 10.3390/molecules28155632. Molecules. 2023. PMID: 37570616 Free PMC article.
-
Synthesis of 4-oxotetrahydropyrimidine-1(2H)-carboxamides derivatives as capsid assembly modulators of hepatitis B virus.Med Chem Res. 2021;30(2):459-472. doi: 10.1007/s00044-020-02677-3. Epub 2021 Jan 11. Med Chem Res. 2021. PMID: 33456291 Free PMC article.
-
Discovery of New Small Molecule Hits as Hepatitis B Virus Capsid Assembly Modulators: Structure and Pharmacophore-Based Approaches.Viruses. 2021 Apr 27;13(5):770. doi: 10.3390/v13050770. Viruses. 2021. PMID: 33925540 Free PMC article.
-
Current Progress in the Development of Hepatitis B Virus Capsid Assembly Modulators: Chemical Structure, Mode-of-Action and Efficacy.Molecules. 2021 Dec 7;26(24):7420. doi: 10.3390/molecules26247420. Molecules. 2021. PMID: 34946502 Free PMC article. Review.
References
-
- Global Hepatitis Report, http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?... (2017).
-
- Chan SL; Wong VWS; Qin SK; Chan HLY, Infection and Cancer: The Case of Hepatitis B. J. Clin. Oncol 34 (2016) 83–90. - PubMed
-
- Cheng YC, L-Nucleoside Analogues as a Novel Class of HBV Drugs. Antiviral Ther. 7 (2002) L83–L83.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

