Adipose stem cell crosstalk with chemo-residual breast cancer cells: implications for tumor recurrence
- PMID: 30594967
- PMCID: PMC6422973
- DOI: 10.1007/s10549-018-05103-w
Adipose stem cell crosstalk with chemo-residual breast cancer cells: implications for tumor recurrence
Abstract
Purpose: Most triple-negative breast cancer (TNBC) patients exhibit an incomplete response to neoadjuvant chemotherapy, resulting in chemo-residual tumor cells that drive tumor recurrence and patient mortality. Accordingly, strategies for eliminating chemo-residual tumor cells are urgently needed. Although stromal cells contribute to tumor cell invasion, to date, their ability to influence chemo-residual tumor cell behavior has not been examined. Our study is the first to investigate cross-talk between adipose-derived stem cells (ASCs) and chemo-residual TNBC cells. We examine if ASCs promote chemo-residual tumor cell proliferation, having implications for tumor recurrence.
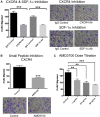
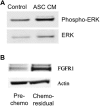
Methods: ASC migration toward chemo-residual TNBC cells was tested in a transwell migration assay. Importance of the SDF-1α/CXCR4 axis was determined using neutralizing antibodies and a small molecule inhibitor. The ability of ASCs to drive tumor cell proliferation was analyzed by culturing tumor cells ± ASC conditioned media (CM) and determining cell counts. Downstream signaling pathways activated in chemo-residual tumor cells following their exposure to ASC CM were studied by immunoblotting. Importance of FGF2 in promoting proliferation was assessed using an FGF2-neutralizing antibody.
Results: ASCs migrated toward chemo-residual TNBC cells in a CXCR4/SDF-1α-dependent manner. Moreover, ASC CM increased chemo-residual tumor cell proliferation and activity of extracellular signal-regulated kinase (ERK). An FGF2-neutralizing antibody inhibited ASC-induced chemo-residual tumor cell proliferation.
Conclusions: ASCs migrate toward chemo-residual TNBC cells via SDF-1α/CXCR4 signaling, and drive chemo-residual tumor cell proliferation in a paracrine manner by secreting FGF2 and activating ERK. This paracrine signaling can potentially be targeted to prevent tumor recurrence.
Keywords: Adipose-derived stem cells (ASCs); Fibroblast growth factor 2 (FGF2); Migration; Proliferation; Recurrence; Triple-negative breast cancer (TNBC).
Conflict of interest statement
Conflict of interest
No authors on this manuscript declare a conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures



Similar articles
-
Chemotherapy enriches for an invasive triple-negative breast tumor cell subpopulation expressing a precursor form of N-cadherin on the cell surface.Oncotarget. 2016 Dec 20;7(51):84030-84042. doi: 10.18632/oncotarget.12767. Oncotarget. 2016. PMID: 27768598 Free PMC article.
-
Nuclear basic fibroblast growth factor regulates triple-negative breast cancer chemo-resistance.Breast Cancer Res. 2015 Jul 4;17(1):91. doi: 10.1186/s13058-015-0590-3. Breast Cancer Res. 2015. PMID: 26141457 Free PMC article.
-
LL-37 stimulates the functions of adipose-derived stromal/stem cells via early growth response 1 and the MAPK pathway.Stem Cell Res Ther. 2016 Apr 19;7(1):58. doi: 10.1186/s13287-016-0313-4. Stem Cell Res Ther. 2016. PMID: 27095351 Free PMC article.
-
CXCR4/CXCL12 blockade therapy; a new horizon in TNBC therapy.Med Oncol. 2025 Apr 11;42(5):161. doi: 10.1007/s12032-025-02705-5. Med Oncol. 2025. PMID: 40216617 Review.
-
The Crosstalk Between Adipose-Derived Stem or Stromal Cells (ASC) and Cancer Cells and ASC-Mediated Effects on Cancer Formation and Progression-ASCs: Safety Hazard or Harmless Source of Tropism?Stem Cells Transl Med. 2022 Apr 29;11(4):394-406. doi: 10.1093/stcltm/szac002. Stem Cells Transl Med. 2022. PMID: 35274703 Free PMC article. Review.
Cited by
-
Exploring the multifaceted role of obesity in breast cancer progression.Front Cell Dev Biol. 2024 Jul 8;12:1408844. doi: 10.3389/fcell.2024.1408844. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39040042 Free PMC article. Review.
-
The Importance of Breast Adipose Tissue in Breast Cancer.Int J Mol Sci. 2020 Aug 11;21(16):5760. doi: 10.3390/ijms21165760. Int J Mol Sci. 2020. PMID: 32796696 Free PMC article. Review.
-
Individual Variabilities in Adipose Stem Cell Proliferation, Gene Expression and Responses to Lipopolysaccharide Stimulation.Int J Mol Sci. 2022 Oct 19;23(20):12534. doi: 10.3390/ijms232012534. Int J Mol Sci. 2022. PMID: 36293398 Free PMC article.
-
Peritoneal adipose stem cell-derived extracellular vesicles mediate the regulation of ovarian cancer cell proliferation and migration through EGFR-NF-κB signaling.Genes Dis. 2024 Mar 28;12(2):101283. doi: 10.1016/j.gendis.2024.101283. eCollection 2025 Mar. Genes Dis. 2024. PMID: 39759123 Free PMC article.
-
Role of the CXCR4-LASP1 Axis in the Stabilization of Snail1 in Triple-Negative Breast Cancer.Cancers (Basel). 2020 Aug 21;12(9):2372. doi: 10.3390/cancers12092372. Cancers (Basel). 2020. PMID: 32825729 Free PMC article.
References
-
- Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clinical Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. - DOI - PubMed
-
- Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

