De Novo Mutations Affecting the Catalytic Cα Subunit of PP2A, PPP2CA, Cause Syndromic Intellectual Disability Resembling Other PP2A-Related Neurodevelopmental Disorders
- PMID: 30595372
- PMCID: PMC6323609
- DOI: 10.1016/j.ajhg.2018.12.002
De Novo Mutations Affecting the Catalytic Cα Subunit of PP2A, PPP2CA, Cause Syndromic Intellectual Disability Resembling Other PP2A-Related Neurodevelopmental Disorders
Erratum in
-
De Novo Mutations Affecting the Catalytic Cα Subunit of PP2A, PPP2CA, Cause Syndromic Intellectual Disability Resembling Other PP2A-Related Neurodevelopmental Disorders.Am J Hum Genet. 2019 Feb 7;104(2):357. doi: 10.1016/j.ajhg.2019.01.003. Am J Hum Genet. 2019. PMID: 30735662 Free PMC article. No abstract available.
Abstract
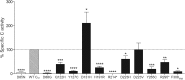
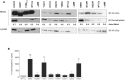
Type 2A protein phosphatases (PP2As) are highly expressed in the brain and regulate neuronal signaling by catalyzing phospho-Ser/Thr dephosphorylations in diverse substrates. PP2A holoenzymes comprise catalytic C-, scaffolding A-, and regulatory B-type subunits, which determine substrate specificity and physiological function. Interestingly, de novo mutations in genes encoding A- and B-type subunits have recently been implicated in intellectual disability (ID) and developmental delay (DD). We now report 16 individuals with mild to profound ID and DD and a de novo mutation in PPP2CA, encoding the catalytic Cα subunit. Other frequently observed features were severe language delay (71%), hypotonia (69%), epilepsy (63%), and brain abnormalities such as ventriculomegaly and a small corpus callosum (67%). Behavioral problems, including autism spectrum disorders, were reported in 47% of individuals, and three individuals had a congenital heart defect. PPP2CA de novo mutations included a partial gene deletion, a frameshift, three nonsense mutations, a single amino acid duplication, a recurrent mutation, and eight non-recurrent missense mutations. Functional studies showed complete PP2A dysfunction in four individuals with seemingly milder ID, hinting at haploinsufficiency. Ten other individuals showed mutation-specific biochemical distortions, including poor expression, altered binding to the A subunit and specific B-type subunits, and impaired phosphatase activity and C-terminal methylation. Four were suspected to have a dominant-negative mechanism, which correlated with severe ID. Two missense variants affecting the same residue largely behaved as wild-type in our functional assays. Overall, we found that pathogenic PPP2CA variants impair PP2A-B56(δ) functionality, suggesting that PP2A-related neurodevelopmental disorders constitute functionally converging ID syndromes.
Keywords: PP2A; PP2A-related neurodevelopmental disorders; PPP2CA; de novo mutation; epilepsy; intellectual disability; syndrome.
Copyright © 2018 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Vissers L.E., Gilissen C., Veltman J.A. Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 2016;17:9–18. - PubMed
-
- de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. - PubMed
-
- Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. - PubMed
-
- Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

