Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting β Cell Proliferation and Function in Obesity
- PMID: 30595478
- PMCID: PMC6701710
- DOI: 10.1016/j.cmet.2018.12.003
Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting β Cell Proliferation and Function in Obesity
Abstract
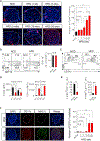
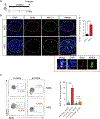
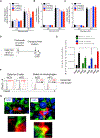
The nature of obesity-associated islet inflammation and its impact on β cell abnormalities remains poorly defined. Here, we explore immune cell components of islet inflammation and define their roles in regulating β cell function and proliferation. Islet inflammation in obese mice is dominated by macrophages. We identify two islet-resident macrophage populations, characterized by their anatomical distributions, distinct phenotypes, and functional properties. Obesity induces the local expansion of resident intra-islet macrophages, independent of recruitment from circulating monocytes. Functionally, intra-islet macrophages impair β cell function in a cell-cell contact-dependent manner. Increased engulfment of β cell insulin secretory granules by intra-islet macrophages in obese mice may contribute to restricting insulin secretion. In contrast, both intra- and peri-islet macrophage populations from obese mice promote β cell proliferation in a PDGFR signaling-dependent manner. Together, these data define distinct roles and mechanisms for islet macrophages in the regulation of islet β cells.
Keywords: islet inflammation; local macrophages proliferation; macrophages; obesity; β cell function; β cell proliferation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Gaining Weight: Insulin-Eating Islet Macrophages.Immunity. 2019 Jan 15;50(1):13-15. doi: 10.1016/j.immuni.2018.12.026. Immunity. 2019. PMID: 30650372
-
Drivers of islet inflammation.Nat Rev Endocrinol. 2019 Mar;15(3):128. doi: 10.1038/s41574-019-0159-9. Nat Rev Endocrinol. 2019. PMID: 30655623 No abstract available.
Similar articles
-
The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities.Nat Rev Endocrinol. 2020 Feb;16(2):81-90. doi: 10.1038/s41574-019-0286-3. Epub 2019 Dec 13. Nat Rev Endocrinol. 2020. PMID: 31836875 Free PMC article. Review.
-
GPR92 activation in islet macrophages controls β cell function in a diet-induced obesity model.J Clin Invest. 2022 Nov 1;132(21):e160097. doi: 10.1172/JCI160097. J Clin Invest. 2022. PMID: 36066975 Free PMC article.
-
Adipose Tissue Macrophages Modulate Obesity-Associated β Cell Adaptations through Secreted miRNA-Containing Extracellular Vesicles.Cells. 2021 Sep 17;10(9):2451. doi: 10.3390/cells10092451. Cells. 2021. PMID: 34572101 Free PMC article.
-
Accumulation of microbial DNAs promotes to islet inflammation and β cell abnormalities in obesity in mice.Nat Commun. 2022 Jan 28;13(1):565. doi: 10.1038/s41467-022-28239-2. Nat Commun. 2022. PMID: 35091566 Free PMC article.
-
Crosstalk between Macrophages and Pancreatic β-Cells in Islet Development, Homeostasis and Disease.Int J Mol Sci. 2021 Feb 10;22(4):1765. doi: 10.3390/ijms22041765. Int J Mol Sci. 2021. PMID: 33578952 Free PMC article. Review.
Cited by
-
The immunology of sickness metabolism.Cell Mol Immunol. 2024 Sep;21(9):1051-1065. doi: 10.1038/s41423-024-01192-4. Epub 2024 Aug 6. Cell Mol Immunol. 2024. PMID: 39107476 Free PMC article. Review.
-
Pancreatic Crosstalk in the Disease Setting: Understanding the Impact of Exocrine Disease on Endocrine Function.Compr Physiol. 2024 Mar 29;14(2):5371-5387. doi: 10.1002/cphy.c230008. Compr Physiol. 2024. PMID: 39109973 Free PMC article. Review.
-
The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications-Update in Pathogenesis, Treatment and Monitoring.Cells. 2020 Aug 11;9(8):1877. doi: 10.3390/cells9081877. Cells. 2020. PMID: 32796572 Free PMC article. Review.
-
The roles of tissue-resident macrophages in sepsis-associated organ dysfunction.Heliyon. 2023 Oct 30;9(11):e21391. doi: 10.1016/j.heliyon.2023.e21391. eCollection 2023 Nov. Heliyon. 2023. PMID: 38027963 Free PMC article. Review.
-
Polyene Phosphatidylcholine Ameliorates High Fat Diet-Induced Non-alcoholic Fatty Liver Disease via Remodeling Metabolism and Inflammation.Front Physiol. 2022 Feb 28;13:810143. doi: 10.3389/fphys.2022.810143. eCollection 2022. Front Physiol. 2022. PMID: 35295576 Free PMC article.
References
-
- Araújo TG, Oliveira AG, Carvalho BM, Guadagnini D, Protzek AO, Carvalheira JB, Boschero AC, and Saad MJ (2012). Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Endocrinology 153, 5760–5769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

