Optogenetic Delineation of Receptor Tyrosine Kinase Subcircuits in PC12 Cell Differentiation
- PMID: 30595532
- PMCID: PMC7372508
- DOI: 10.1016/j.chembiol.2018.11.004
Optogenetic Delineation of Receptor Tyrosine Kinase Subcircuits in PC12 Cell Differentiation
Abstract
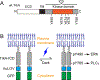
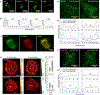
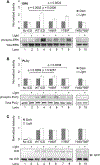
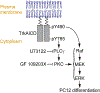
Nerve growth factor elicits signaling outcomes by interacting with both its high-affinity receptor, TrkA, and its low-affinity receptor, p75NTR. Although these two receptors can regulate distinct cellular outcomes, they both activate the extracellular-signal-regulated kinase pathway upon nerve growth factor stimulation. To delineate TrkA subcircuits in PC12 cell differentiation, we developed an optogenetic system whereby light was used to specifically activate TrkA signaling in the absence of nerve growth factor. By using tyrosine mutants of the optogenetic TrkA in combination with pathway-specific pharmacological inhibition, we find that Y490 and Y785 each contributes to PC12 cell differentiation through the extracellular-signal-regulated kinase pathway in an additive manner. Optogenetic activation of TrkA eliminates the confounding effect of p75NTR and other potential off-target effects of the ligand. This approach can be generalized for the mechanistic study of other receptor-mediated signaling pathways.
Keywords: ERK; PC12 cell differentiation; PLCγ; TrkA signaling subcircuits; Y490; Y785; nerve growth factor; optogenetics.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Discoidin domain receptor 1 (DDR1) signaling in PC12 cells: activation of juxtamembrane domains in PDGFR/DDR/TrkA chimeric receptors.FASEB J. 2000 May;14(7):973-81. doi: 10.1096/fasebj.14.7.973. FASEB J. 2000. PMID: 10783152
-
Inhibition of Abl tyrosine kinase enhances nerve growth factor-mediated signaling in Bcr-Abl transformed cells via the alteration of signaling complex and the receptor turnover.Oncogene. 2008 Aug 7;27(34):4678-89. doi: 10.1038/onc.2008.107. Epub 2008 Apr 21. Oncogene. 2008. PMID: 18427551
-
Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75.Neuroscience. 2002;115(4):1089-108. doi: 10.1016/s0306-4522(02)00539-0. Neuroscience. 2002. PMID: 12453482
-
NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
-
Signaling pathways for PC12 cell differentiation: making the right connections.Science. 2002 May 31;296(5573):1648-9. doi: 10.1126/science.1071552. Science. 2002. PMID: 12040181 Review.
Cited by
-
Optogenetic engineering for ion channel modulation.Curr Opin Chem Biol. 2025 Apr;85:102569. doi: 10.1016/j.cbpa.2025.102569. Epub 2025 Feb 3. Curr Opin Chem Biol. 2025. PMID: 39903997 Review.
-
Alternating Cellular Functions by Optogenetic Control of Organelles.Methods Mol Biol. 2025;2840:175-183. doi: 10.1007/978-1-0716-4047-0_13. Methods Mol Biol. 2025. PMID: 39724352
-
Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila.Elife. 2020 Oct 6;9:e57395. doi: 10.7554/eLife.57395. Elife. 2020. PMID: 33021199 Free PMC article.
-
Functional Modulation of Receptor Proteins on Cellular Interface with Optogenetic System.Adv Exp Med Biol. 2021;1293:247-263. doi: 10.1007/978-981-15-8763-4_15. Adv Exp Med Biol. 2021. PMID: 33398818 Review.
-
Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions.Neural Regen Res. 2022 Jan;17(1):25-30. doi: 10.4103/1673-5374.314293. Neural Regen Res. 2022. PMID: 34100422 Free PMC article.
References
-
- Bae SS, Lee YH, Chang JS, Galadari SH, Kim YS, Ryu SH, and Suh PG (1998). Src homology domains of phospholipase C gamma 1 inhibit nerve growth factor-induced differentiation of PC12 cells. Journal of neurochemistry 71, 178–185. - PubMed
-
- Barker PA (1998). p75NTR: A study in contrasts. Cell death and differentiation 5, 346–356. - PubMed
-
- Bothwell M (1995). Functional interactions of neurotrophins and neurotrophin receptors. Annual review of neuroscience 18, 223–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

