Anti-TNF- α Therapy Suppresses Proinflammatory Activities of Mucosal Neutrophils in Inflammatory Bowel Disease
- PMID: 30595666
- PMCID: PMC6282128
- DOI: 10.1155/2018/3021863
Anti-TNF- α Therapy Suppresses Proinflammatory Activities of Mucosal Neutrophils in Inflammatory Bowel Disease
Abstract
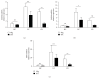
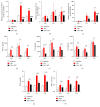
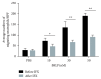
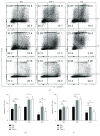
Neutrophils have been found to play an important role in the pathogenesis of inflammatory bowel disease (IBD), and anti-TNF-α mAb (i.e., infliximab) therapy is demonstrated to be effective in the induction of clinical remission and mucosal healing in these patients. However, how anti-TNF-α mAb regulates the functions of neutrophils is still unknown. Herein, we found that anti-TNF-α therapy significantly downregulated infiltration of neutrophils in inflamed mucosa of IBD patients. Importantly, anti-TNF-α mAb could inhibit neutrophils to produce proinflammatory mediators, such as ROS, calprotectin, IL-8, IL-6, and TNF-α. These data indicate that TNF-α plays a critical role in the induction of mucosal inflammatory response, and that blockade of TNF-α modulates intestinal homeostasis through balancing immune responses of neutrophils.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

