Highly sensitive LC-MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
- PMID: 30595944
- PMCID: PMC6308917
- DOI: 10.1016/j.jpha.2017.06.004
Highly sensitive LC-MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
Abstract
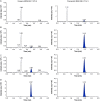
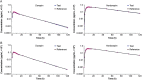
A selective, sensitive and rugged liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay has been developed for the simultaneous determination of doxepin (Dox) and its pharmacologically active metabolite, nordoxepin (NDox) in human plasma. The analytes and their internal standards (IS) were extracted from 500 µL of human plasma by liquid-liquid extraction using methyl tert-butyl ether. Chromatographic separation was achieved on Hypurity C8 column (100 mm × 4.6 mm, 5 µm) using a mixture of acetonitrile-methanol (95:5, v/v) and 2.0 mM ammonium formate in 93:7 (v/v) ratio. Detection was accomplished by tandem mass spectrometry in the positive ionization and multiple reaction monitoring acquisition mode. The protonated precursor to product ion transitions studied for Dox, NDox, and their corresponding ISs, propranolol and desipramine, were m/z 280.1→107.0, 266.0 →107.0, 260.1→116.1 and 267.1→72.1, respectively. A linear dynamic range of 15.0-3900 pg/mL for Dox and 5.00-1300 pg/mL for NDox was established with mean correlation coefficient (r 2) of 0.9991 and 0.9993, respectively. The extraction recovery ranged from 86.6%-90.4% and 88.0%-99.1% for Dox and NDox, respectively. The intra-batch and inter-batch precision (% CV) across quality control levels was ≤ 8.3% for both the analytes. Stability evaluated under different storage conditions showed no evidence of degradation and the % change in stability samples compared to nominal concentration ranged from 4.7% to 12.3%. The method was successfully applied to a bioequivalence study of 6 mg doxepin hydrochloride orally disintegrating tablet in 41 healthy Indian subjects under fasting and fed conditions.
Keywords: Bioequivalence study; Doxepin; Human plasma; LC–MS/MS; Liquid-liquid extraction; Nordoxepin.
Figures

Similar articles
-
High-throughput protein precipitation method with 96-well plate for determination of doxepin and nordoxepin in human plasma using LC-MS/MS.Biomed Chromatogr. 2020 Aug;34(8):e4844. doi: 10.1002/bmc.4844. Epub 2020 Apr 23. Biomed Chromatogr. 2020. PMID: 32250456
-
Analysis of second-generation antidepressant drug, sertraline and its active metabolite, N-desmethyl sertraline in human plasma by a sensitive and selective liquid chromatography-tandem mass spectrometry method.J Chromatogr B Analyt Technol Biomed Life Sci. 2009 Jan 15;877(3):221-9. doi: 10.1016/j.jchromb.2008.12.008. Epub 2008 Dec 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2009. PMID: 19109078
-
Simultaneous analysis of oxybutynin and its active metabolite N-desethyl oxybutynin in human plasma by stable isotope dilution LC-MS/MS to support a bioequivalence study.J Pharm Biomed Anal. 2013 Oct;84:244-55. doi: 10.1016/j.jpba.2013.06.024. Epub 2013 Jun 28. J Pharm Biomed Anal. 2013. PMID: 23867086
-
Quantitation of donepezil and its active metabolite 6-O-desmethyl donepezil in human plasma by a selective and sensitive liquid chromatography-tandem mass spectrometric method.Anal Chim Acta. 2008 Nov 23;629(1-2):145-57. doi: 10.1016/j.aca.2008.09.048. Epub 2008 Oct 1. Anal Chim Acta. 2008. PMID: 18940331
-
High throughput and sensitive determination of trazodone and its primary metabolite, m-chlorophenylpiperazine, in human plasma by liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Aug 1;871(1):44-54. doi: 10.1016/j.jchromb.2008.06.046. Epub 2008 Jul 3. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18621591
Cited by
-
Stir bar sorptive-dispersive microextraction by a poly(methacrylic acid-co-ethylene glycol dimethacrylate)-based magnetic sorbent for the determination of tricyclic antidepressants and their main active metabolites in human urine.Mikrochim Acta. 2022 Jan 8;189(2):52. doi: 10.1007/s00604-021-05156-7. Mikrochim Acta. 2022. PMID: 35000010 Free PMC article.
-
Determination of asenapine in presence of its inactive metabolites in human plasma by LC-MS/MS.J Pharm Anal. 2018 Oct;8(5):341-347. doi: 10.1016/j.jpha.2018.06.002. Epub 2018 Jun 18. J Pharm Anal. 2018. PMID: 30345149 Free PMC article.
References
-
- Akhondzadeh S., Faraji H., Sadeghi M. Double-blind comparison of fluoxetine and nortriptyline in the treatment of moderate to severe major depression. J. Clin. Pharm. Ther. 2003;28:379–384. - PubMed
-
- Kandagal P.B., Ashoka S., Seetharamappa J. Study of interaction between doxepin and human serum albumin by spectroscopic methods. J. Photochem. Photobiol. A: Chem. 2006;179:161–166.
-
- Katwala J., Kumar A.K., Sejpal J.J. Therapeutic rationale for low dose doxepin in insomnia patients. Asian Pac. J. Trop. Dis. 2013;3:331–336.
-
- Schomburg R., Remane D., Fassbender K. Doxepin concentrations in plasma and cerebrospinal fluid. J. Neural Transm. 2011;118:641–645. - PubMed
-
- Gronewold A., Dettling A., Haffner H.T. Doxepin and nordoxepin concentrations in body fluids and tissues in doxepin associated deaths. Forensic Sci. Int. 2009;190:74–79. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

