Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt (EDTA-Na2) solution for catheter locks
- PMID: 30595945
- PMCID: PMC6308019
- DOI: 10.1016/j.jpha.2017.09.004
Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt (EDTA-Na2) solution for catheter locks
Abstract
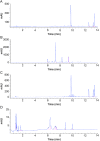
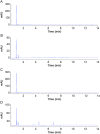
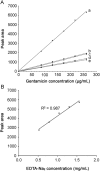
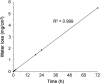
A lock solution composed of gentamicin sulfate (5 mg/mL) and ethylenediaminetetraacetic acid disodium salt (EDTA-Na2, 30 mg/mL) could fully eradicate in vivo bacterial biofilms in totally implantable venous access ports (TIVAP). In this study, fabrication, conditioning and sterilization processes of antimicrobial lock solution (ALS) were detailed and completed by a stability study. Stability of ALS was conducted for 12 months in vial (25 °C ± 2 °C, 60% ± 5% relative humidity (RH), and at 40 °C ± 2 °C, RH 75% ± 5%) and for 24 h and 72 h in TIVAP (40 °C ± 2 °C, RH 75% ± 5%). A stability indicating HPLC assay with UV detection for simultaneous quantification of gentamicin sulfate and EDTA-Na2 was developed. ALS was assayed by ion-pairing high performance liquid chromatography (HPLC) needing gentamicin derivatization, EDTA-Na2 metallocomplexation of samples and gradient mobile phase. HPLC methods to separate four gentamicin components and EDTA-Na2 were validated. Efficiency of sterility procedure and conditioning of ALS was confirmed by bacterial endotoxins and sterility tests. Physicochemical stability of ALS was determined by visual inspection, osmolality, pH, and sub-visible particle counting. Results confirmed that the stability of ALS in vials was maintained for 12 months and 24 h and 72 h in TIVAP.
Keywords: Gentamicin-EDTA-Na2 loaded antimicrobial lock solution; Pharmaceutical compounding; Stability indicating HPLC assay method; Totally implantable venous access ports.
Figures
Similar articles
-
A validated reverse phase HPLC method for the determination of disodium EDTA in meropenem drug substance with UV-detection using precolumn derivatization technique.Anal Chem Insights. 2011;6:7-14. doi: 10.4137/ACI.S5953. Epub 2011 Feb 23. Anal Chem Insights. 2011. PMID: 21760705 Free PMC article.
-
Stability of 10 mg/mL cefuroxime solution for intracameral injection in commonly used polypropylene syringes and new ready-to-use cyclic olefin copolymer sterile vials using the LC-UV stability-indicating method.Drug Dev Ind Pharm. 2016 Jan;42(1):166-174. doi: 10.3109/03639045.2015.1038273. Epub 2015 May 26. Drug Dev Ind Pharm. 2016. PMID: 26006333
-
Physical Compatibility, Antimicrobial Activity, and Stability of Cefazolin Combined with Gentamicin or Ethanol in Sodium Citrate as a Catheter Lock Solution.J Pharm Bioallied Sci. 2021 Jul-Sep;13(3):298-304. doi: 10.4103/jpbs.JPBS_619_20. Epub 2021 Nov 24. J Pharm Bioallied Sci. 2021. PMID: 35017885 Free PMC article.
-
pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms.J Infect Dis. 2014 Nov 1;210(9):1357-66. doi: 10.1093/infdis/jiu286. Epub 2014 May 15. J Infect Dis. 2014. PMID: 24837402
-
Management of infections related to totally implantable venous-access ports: challenges and perspectives.Lancet Infect Dis. 2014 Feb;14(2):146-59. doi: 10.1016/S1473-3099(13)70266-4. Epub 2013 Dec 5. Lancet Infect Dis. 2014. PMID: 24314751 Review.
Cited by
-
Higher Brain Uptake of Gentamicin and Ceftazidime under Isoflurane Anesthesia Compared to Ketamine/Xylazine.Pharmaceutics. 2024 Jan 19;16(1):135. doi: 10.3390/pharmaceutics16010135. Pharmaceutics. 2024. PMID: 38276505 Free PMC article.
-
Biofilm Resilience: Molecular Mechanisms Driving Antibiotic Resistance in Clinical Contexts.Biology (Basel). 2025 Feb 6;14(2):165. doi: 10.3390/biology14020165. Biology (Basel). 2025. PMID: 40001933 Free PMC article. Review.
References
-
- Raad I., Hanna H., Maki D. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect. Dis. 2007;7:645–657. - PubMed
-
- Beloin C., Fernández-Hidalgo N., Lebeaux D. Understanding biofilm formation in intravascular device-related infections. Intensive Care Med. 2017;43:443–446. - PubMed
-
- Bookstaver P.B., Rokas K.E.E., Norris L.B. Vol. 70. 2013. Stability and compatibility of antimicrobial lock solutions; pp. 2185–2198. (Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm.). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

