In vivo antioxidant activity of mackerel (Scomber japonicus) muscle protein hydrolysate
- PMID: 30595992
- PMCID: PMC6305115
- DOI: 10.7717/peerj.6181
In vivo antioxidant activity of mackerel (Scomber japonicus) muscle protein hydrolysate
Abstract
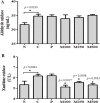
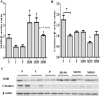
Pacific chub mackerel (Scomber japonicus) is an important fish throughout the world, especially in East Asian countries, including Korea, China, and Japan. Protein hydrolysates from marine sources are commonly used as nutritional supplements, functional ingredients, and flavor enhancers in the food, beverage, and pharmaceutical industries. Antioxidants isolated from fish are relatively easy to prepare, are cost effective, and have no reported side effects. Hence, the present study aimed to investigate the in vivo antioxidant activities of mackerel muscle protein hydrolysate (MMPH) prepared using Protamex. The in vivo bioactivities of MMPH were investigated in alcoholic fatty liver mice (C57BL/6). Serum alanine aminotransferase and aspartate aminotransferase levels were comparable in test and control mice, whereas serum triglyceride and lipid peroxidation levels significantly (p < 0.05; p < 0.001) decreased after administration of MMPH (100-500 mg kg-1), especially at a concentration of 100 mg kg-1. A significant (p < 0.05) reduction in xanthine oxidase activity was observed in all groups treated with MMPH (100-500 mg kg-1), as compared with the control group. Significantly (p < 0.05) higher superoxide dismutase (SOD) activity/protein expression and regulated catalase (CAT) activity/protein expression levels were observed in groups administered MMPH (100-500 mg kg-1), especially at a concentration of 100 mg kg-1. These results show that the abundant amino acids of S. japonicus play an important role in the cytosol of the liver cells by directly participating in the expression of xanthine oxidase and the detoxifying SOD and CAT proteins, thereby enhancing antioxidant ability and ultimately, inhibiting lipid peroxidation. This study demonstrated that muscle protein hydrolysate from S. japonicus has strong antioxidant activities.
Keywords: Enzymatic hydrolysis; In vivo antioxidant activity; Protamex; Protein Hydrolysate; SOD and CAT protein expression.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
-
- Ali B, Naima NA, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chemistry. 2010;118:559–565. doi: 10.1016/j.foodchem.2009.05.021. - DOI
-
- Atawodi SE. Evaluation of the hypoglycemic, hypolipidemic and antioxidant effects of methanolic extract of ‘Ata-Ofa’ polyherbal tea (A-polyherbal) in alloxan-induced diabetic rats. Drug Invention Today. 2011;3:270–276.
-
- Bashir KMI, Kim M-S, Stahl U, Cho M-G. Agrobacterium-mediated genetic transformation of Dictyosphaerium pulchellum for the expression of erythropoietin. Journal of Applied Phycology. 2018b doi: 10.1007/s10811-018-1483-5. In Press. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

