Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review
- PMID: 30596158
- PMCID: PMC6305839
- DOI: 10.1016/j.bioactmat.2018.12.003
Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Bioact Mater. 2020 Dec 4;6(6):1789-1790. doi: 10.1016/j.bioactmat.2020.11.009. eCollection 2021 Jun. Bioact Mater. 2020. PMID: 33336111 Free PMC article.
Abstract
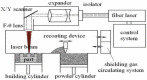
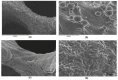
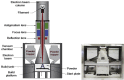
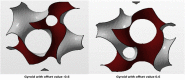
Recently, the fabrication methods of orthopedic implants and devices have been greatly developed. Additive manufacturing technology allows the production of complex structures with bio-mimicry features, and has the potential to overcome the limitations of conventional fabrication methods. This review explores open-cellular structural design for porous metal implant applications, in relation to the mechanical properties, biocompatibility, and biodegradability. Several types of additive manufacturing techniques including selective laser sintering, selective laser melting, and electron beam melting, are discussed for different applications. Additive manufacturing through powder bed fusion shows great potential for the fabrication of high-quality porous metal implants. However, the powder bed fusion technique still faces two major challenges: it is high cost and time-consuming. In addition, triply periodic minimal surface (TPMS) structures are also analyzed in this paper, targeting the design of metal implants with an enhanced biomorphic environment.
Keywords: Additive manufacturing; Porosity; Powder bed fusion; TPMS structures.
Figures









References
-
- Palmer L.C., Newcomb C.J., Kaltz S.R., Spoerke E.D., Stupp S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008;108:4754–4783. https://pubs.acs.org/doi/abs/10.1021/cr8004422 - DOI - PMC - PubMed
-
- Goods Administration Therapeutic. 2011. Australian Regulatory Guidelines for Medical Devices.https://www.tga.gov.au/sites/default/files/devices-argmd-01.pdf
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

