Downregulation of NEAT1 reverses the radioactive iodine resistance of papillary thyroid carcinoma cell via miR-101-3p/FN1/PI3K-AKT signaling pathway
- PMID: 30596336
- PMCID: PMC6343742
- DOI: 10.1080/15384101.2018.1560203
Downregulation of NEAT1 reverses the radioactive iodine resistance of papillary thyroid carcinoma cell via miR-101-3p/FN1/PI3K-AKT signaling pathway
Retraction in
-
Statement of Retraction: Downregulation of NEAT1 reverses the radioactive iodine resistance of papillary thyroid carcinoma cell via miR-101-3p/FN1/PI3K-AKT signaling pathway.Cell Cycle. 2022 Dec;21(23):2550. doi: 10.1080/15384101.2022.2097797. Epub 2022 Jul 15. Cell Cycle. 2022. PMID: 35838519 Free PMC article. No abstract available.
Abstract
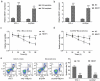
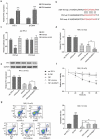
Considering the resistance of papillary thyroid cancer (PTC) 131I therapy, this study was designed to find a solution at molecular respect. By probing into lncRNA-NEAT1/miR-101-3p/FN1 axis and PI3K/AKT signaling pathway, this study provided a potential target for PTC therapy. 131I-resistant cell lines were established by continuous treatment with median-lethal 131I. Bioinformatic analysis was applied to filtrate possible lncRNA/miRNA/mRNA and related signaling pathway. Luciferase reporter assay was employed in the verification of the targeting relationship between lncRNA and miRNA as well as miRNA and mRNA. MTT assay and flow cytometry assay were performed to observe the impact of NEAT1/miR-101-3p/FN1 on cell viability and apoptosis in radioactivity iodine (RAI)-resistant PTC cell lines, respectively. Western blot and qRT-PCR were conducted to measure the expression of proteins and mRNAs in RAI-resistant PTC tissues and cells. Meanwhile, endogenous PTC mice model were constructed, in order to verify the relation between NEAT1 and RAI-resistance in vivo. NEAT1 was over-expressed in RAI-resistant PTC tissues and cell lines and could resist RAI by accelerating proliferation accompanied by suppressing apoptosis. It indicated that overexpressed NEAT1 restrained the damage of RAI to tumor in both macroscopic and microcosmic. Besides, NEAT1/miR-101-3p exhibited a negative correlation by directly targeting each other. The expression of FN1, an overexpressed downstream protein in RAI-resistance PTC tissues, could be tuned down by miR-101-3p, while the decrease could be restored by NEAT1. In conclusion, both in vitro and in vivo, NEAT1 suppression could inhibit 131I resistance of PTC by upregulating miR-101-3p/FN1 expression and inactivated PI3K/AKT signaling pathway both in vitro and in vivo.
Keywords: FN1; NEAT1; PI3K/AKT signaling pathway; PTC; RAI-resistance; miR-101-3p.
Figures






References
-
- Zhang H, Cai Y, Zheng L, et al. Long noncoding RNA NEAT1 regulate papillary thyroid cancer progression by modulating miR-129-5p/KLK7 expression. J Cell Physiol. 2018;233:6638–6648. - PubMed
-
- Lupoli R, Cacciapuoti M, Tortora A, et al. Clinical outcome in differentiated thyroid carcinoma and microcarcinoma. Int J Surg. 2014;12(Suppl 1):S148–51. - PubMed
-
- Liu D, Hu S, Hou P, et al. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13:1341–1349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
