The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL-8 secretion
- PMID: 30596396
- PMCID: PMC6237608
- DOI: 10.1111/jcmm.13879
The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL-8 secretion
Abstract
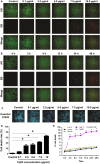
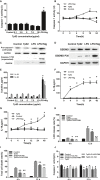
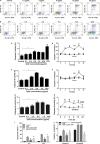
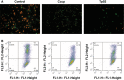
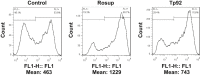
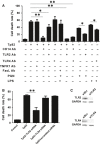
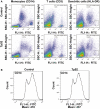
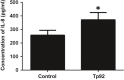
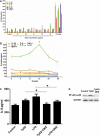
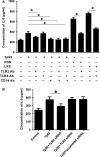
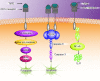
Treponema pallidum is the pathogen that causes syphilis, a sexually transmitted disease; however, the pathogenic mechanism of this organism remains unclear. Tp92 is the only T. pallidum outer membrane protein that has structural features similar to the outer membrane proteins of other Gram-negative bacteria, but the exact functions of this protein remain unknown. In the present study, we demonstrated that the recombinant Tp92 protein can induce human mononuclear cell death. Tp92 mediated the human monocytic cell line derived from an acute monicytic leukemia patient (THP-1) cell death by recognizing CD14 and/or TLR2 on cell surfaces. After the stimulation of THP-1 cells by the Tp92 protein, Tp92 may induce atypical pyroptosis of THP-1 cells via the pro-caspase-1 pathway. Meanwhile, this protein caused the apoptosis of THP-1 cells via the receptor-interacting protein kinase 1/caspase-8/aspase-3 pathway. Tp92 reduced the number of monocytes among peripheral blood mononuclear cells. Interestingly, further research showed that Tp92 failed to increase the tumour necrosis factor-α, interleukin (IL)-1β, IL-6, IL-10, IL-18 and monocyte chemotactic protein 1 (MCP)-1 levels but slightly elevated the IL-8 levels via the Nuclear Factor (NF)-κB pathway in THP-1 cells. The data suggest that Tp92 recognizes CD14 and TLR2, transfers the signal to a downstream pathway, and activates NF-κB to mediate the production of IL-8. This mechanism may help T. pallidum escape recognition and elimination by the host innate immune system.
Keywords: Treponema pallidum; CD14; IL‐8; TLR2; Tp92; apoptosis; membrane protein; pyroptosis.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Salavec M, Bostik V, Kapla J, et al. A repeated syphilis infection imported from Thailand in an HIV positive couple of men‐who‐have‐sex‐with‐men in Czech Republic. Travel Med Infect Dis. 2014;12:84‐87. - PubMed
-
- Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44‐54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

