Direct role of FLT3 in regulation of early lymphoid progenitors
- PMID: 30596405
- PMCID: PMC6492191
- DOI: 10.1111/bjh.15578
Direct role of FLT3 in regulation of early lymphoid progenitors
Abstract
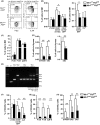
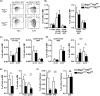
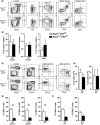
Given that FLT3 expression is highly restricted on lymphoid progenitors, it is possible that the established role of FLT3 in the regulation of B and T lymphopoiesis reflects its high expression and role in regulation of lymphoid-primed multipotent progenitors (LMPPs) or common lymphoid progenitors (CLPs). We generated a Flt3 conditional knock-out (Flt3fl/fl) mouse model to address the direct role of FLT3 in regulation of lymphoid-restricted progenitors, subsequent to turning on Rag1 expression, as well as potentially ontogeny-specific roles in B and T lymphopoiesis. Our studies establish a prominent and direct role of FLT3, independently of the established role of FLT3 in regulation of LMPPs and CLPs, in regulation of fetal as well as adult early B cell progenitors, and the early thymic progenitors (ETPs) in adult mice but not in the fetus. Our findings highlight the potential benefit of targeting poor prognosis acute B-cell progenitor leukaemia and ETP leukaemia with recurrent FLT3 mutations using clinical FLT3 inhibitors.
Keywords: FLT3; conditional knock‐out mouse model; haematopoiesis; lymphoid development; lymphoid progenitors.
© 2018 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Figures

References
-
- Adolfsson, J. , Borge, O.J. , Bryder, D. , Theilgaard‐Monch, K. , Astrand‐Grundstrom, I. , Sitnicka, E. , Sasaki, Y. & Jacobsen, S.E. (2001) Upregulation of Flt3 expression within the bone marrow Lin(‐)Sca1(+)c‐kit(+) stem cell compartment is accompanied by loss of self‐renewal capacity. Immunity, 15, 659–669. - PubMed
-
- Adolfsson, J. , Mansson, R. , Buza‐Vidas, N. , Hultquist, A. , Liuba, K. , Jensen, C.T. , Bryder, D. , Yang, L. , Borge, O.J. , Thoren, L.A. , Anderson, K. , Sitnicka, E. , Sasaki, Y. , Sigvardsson, M. & Jacobsen, S.E. (2005) Identification of Flt3+ lympho‐myeloid stem cells lacking erythro‐megakaryocytic potential a revised road map for adult blood lineage commitment. Cell, 121, 295–306. - PubMed
-
- Almarza, E. , Segovia, J.C. , Guenechea, G. , Gomez, S.G. , Ramirez, A. & Bueren, J.A. (2004) Regulatory elements of the vav gene drive transgene expression in hematopoietic stem cells from adult mice. Experimental Hematology, 32, 360–364. - PubMed
-
- Armstrong, S.A. , Mabon, M.E. , Silverman, L.B. , Li, A. , Gribben, J.G. , Fox, E.A. , Sallan, S.E. & Korsmeyer, S.J. (2004) FLT3 mutations in childhood acute lymphoblastic leukemia. Blood, 103, 3544–3546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

