Symbiont location, host fitness, and possible coadaptation in a symbiosis between social amoebae and bacteria
- PMID: 30596477
- PMCID: PMC6336404
- DOI: 10.7554/eLife.42660
Symbiont location, host fitness, and possible coadaptation in a symbiosis between social amoebae and bacteria
Abstract
Recent symbioses, particularly facultative ones, are well suited for unravelling the evolutionary give and take between partners. Here we look at variation in natural isolates of the social amoeba Dictyostelium discoideum and their relationships with bacterial symbionts, Burkholderia hayleyella and Burkholderia agricolaris. Only about a third of field-collected amoebae carry a symbiont. We cured and cross-infected amoebae hosts with different symbiont association histories and then compared host responses to each symbiont type. Before curing, field-collected clones did not vary significantly in overall fitness, but infected hosts produced morphologically different multicellular structures. After curing and reinfecting, host fitness declined. However, natural B. hayleyella hosts suffered fewer fitness costs when reinfected with B. hayleyella, indicating that they have evolved mechanisms to tolerate their symbiont. Our work suggests that amoebae hosts have evolved mechanisms to tolerate specific acquired symbionts; exploring host-symbiont relationships that vary within species may provide further insights into disease dynamics.
Keywords: Burkholderia; amoebae; dictyostelium; evolutionary biology; infectious disease; microbiology; symbiosis.
© 2018, Shu et al.
Conflict of interest statement
LS, DB, KG, JM, DQ, JS, SD No competing interests declared
Figures
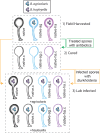




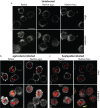



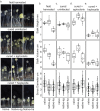
Similar articles
-
The specificity of Burkholderia symbionts in the social amoeba farming symbiosis: Prevalence, species, genetic and phenotypic diversity.Mol Ecol. 2019 Feb;28(4):847-862. doi: 10.1111/mec.14982. Mol Ecol. 2019. PMID: 30575161
-
Fitness costs and benefits vary for two facultative Burkholderia symbionts of the social amoeba, Dictyostelium discoideum.Ecol Evol. 2019 Aug 15;9(17):9878-9890. doi: 10.1002/ece3.5529. eCollection 2019 Sep. Ecol Evol. 2019. PMID: 31534701 Free PMC article.
-
Burkholderia bacteria use chemotaxis to find social amoeba Dictyostelium discoideum hosts.ISME J. 2018 Aug;12(8):1977-1993. doi: 10.1038/s41396-018-0147-4. Epub 2018 May 24. ISME J. 2018. PMID: 29795447 Free PMC article.
-
Symbiotic factors in Burkholderia essential for establishing an association with the bean bug, Riptortus pedestris.Arch Insect Biochem Physiol. 2015 Jan;88(1):4-17. doi: 10.1002/arch.21218. Arch Insect Biochem Physiol. 2015. PMID: 25521625 Review.
-
Riptortus pedestris and Burkholderia symbiont: an ideal model system for insect-microbe symbiotic associations.Res Microbiol. 2017 Apr;168(3):175-187. doi: 10.1016/j.resmic.2016.11.005. Epub 2016 Dec 10. Res Microbiol. 2017. PMID: 27965151 Review.
Cited by
-
Symbiont-Induced Phagosome Changes Rather than Extracellular Discrimination Contribute to the Formation of Social Amoeba Farming Symbiosis.Microbiol Spectr. 2022 Jun 29;10(3):e0172721. doi: 10.1128/spectrum.01727-21. Epub 2022 Apr 20. Microbiol Spectr. 2022. PMID: 35442071 Free PMC article.
-
Tetrahymena predation drives adaptive evolution of Salmonella by disrupting O-antigen biosynthesis and upregulating transcriptional regulator csgD.ISME J. 2025 Jan 2;19(1):wraf070. doi: 10.1093/ismejo/wraf070. ISME J. 2025. PMID: 40226985 Free PMC article.
-
Reduced and Nonreduced Genomes in Paraburkholderia Symbionts of Social Amoebas.mSystems. 2022 Oct 26;7(5):e0056222. doi: 10.1128/msystems.00562-22. Epub 2022 Sep 13. mSystems. 2022. PMID: 36098425 Free PMC article.
-
Loss and resiliency of social amoeba symbiosis under simulated warming.Ecol Evol. 2020 Oct 20;10(23):13182-13189. doi: 10.1002/ece3.6909. eCollection 2020 Dec. Ecol Evol. 2020. PMID: 33304528 Free PMC article.
-
The consequences of viral infection on protists.Commun Biol. 2024 Mar 11;7(1):306. doi: 10.1038/s42003-024-06001-2. Commun Biol. 2024. PMID: 38462656 Free PMC article. Review.
References
-
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67
-
- Bing X, Attardo GM, Vigneron A, Aksoy E, Scolari F, Malacrida A, Weiss BL, Aksoy S. Unravelling the relationship between the tsetse fly and its obligate symbiont Wigglesworthia : transcriptomic and metabolomic landscapes reveal highly integrated physiological networks. Proceedings of the Royal Society B: Biological Sciences. 2017;284:20170360. doi: 10.1098/rspb.2017.0360. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

