Tranexamic acid for patients with nasal haemorrhage (epistaxis)
- PMID: 30596479
- PMCID: PMC6517002
- DOI: 10.1002/14651858.CD004328.pub3
Tranexamic acid for patients with nasal haemorrhage (epistaxis)
Abstract
Background: Epistaxis (nosebleed) most commonly affects children and the elderly. The majority of episodes are managed at home with simple measures. In more severe cases medical intervention is required to either cauterise the bleeding vessel, or to pack the nose with various materials. Tranexamic acid is used in a number of clinical settings to stop bleeding by preventing clot breakdown (fibrinolysis). It may have a role in the management of epistaxis as an adjunct to standard treatments, reducing the need for further intervention.
Objectives: To determine the effects of tranexamic acid (oral, intravenous or topical) compared with placebo, no additional intervention or any other haemostatic agent in the management of patients with epistaxis.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Register (via CRS Web); Central Register of Controlled Trials (CENTRAL) (via CRS Web); PubMed; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 29 October 2018.
Selection criteria: Randomised controlled trials (RCTs) of tranexamic acid (in addition to usual care) compared with usual care plus placebo, usual care alone or usual care plus any other haemostatic agent, to control epistaxis in adults or children.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane. The primary outcomes were control of epistaxis: re-bleeding (as measured by the proportion of patients re-bleeding within a period of up to 10 days) and significant adverse effects (seizures, thromboembolic events). Secondary outcomes were control of epistaxis as measured by the time to stop initial bleeding (the proportion of patients whose bleeding is controlled within a period of up to 30 minutes); severity of re-bleeding (as measured by (a) the proportion of patients requiring any further intervention and (b) the proportion of patients requiring blood transfusion); length of hospital stay and other adverse effects. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
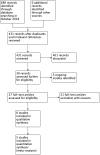
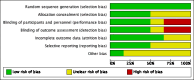
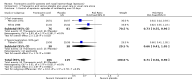
Main results: We included six RCTs (692 participants). The overall risk of bias in the studies was low. Two studies assessed oral administration of tranexamic acid, given regularly over several days, and compared it to placebo. In the other four studies, a single application of topical tranexamic acid was compared with placebo (one study) and a combination of epinephrine and lidocaine or phenylephrine (three studies). All participants were adults.Tranexamic acid versus placeboFor our primary outcome, control of epistaxis: re-bleeding (proportion re-bleeding within 10 days), we were able to pool data from three studies. The pooled result demonstrated a benefit of tranexamic acid compared to placebo, the risk of re-bleeding reducing from 67% to 47% (risk ratio (RR) 0.71, 95% confidence interval (CI) 0.56 to 0.90; three studies; 225 participants; moderate-quality evidence).When we compared the effects of oral and topical tranexamic acid separately the risk of re-bleeding with oral tranexamic acid reduced from 69% to 49%, RR 0.73 (95% CI 0.55 to 0.96; two studies, 157 participants; moderate-quality evidence) and with topical tranexamic acid it reduced from 66% to 43%, RR 0.66 (95% CI 0.41 to 1.05; single study, 68 participants). We rated the quality of evidence provided by the single study as low, therefore it is uncertain whether topical tranexamic acid is effective in stopping bleeding in the 10-day period after a single application.No study specifically sought to identify and report our primary outcome: significant adverse effects (i.e. seizures, thromboembolic events).The secondary outcome time to stop initial bleeding (proportion with bleeding controlled within 30 minutes) was measured in one study using topical tranexamic acid and there was no evidence of a difference at 30 minutes (RR 0.79, 95% CI 0.56 to 1.11; 68 participants; low-quality evidence).No studies reported the proportion of patients requiring any further intervention (e.g. repacking, surgery, embolisation).One study of oral tranexamic acid reported the proportion of patients requiring blood transfusion and found no difference between groups: 5/45 (11%) versus 6/44 (14%) (RR 0.81, 95% CI 0.27 to 2.48; 89 participants; low-quality evidence).Two studies reported hospital length of stay. One study reported a significantly shorter stay in the oral tranexamic acid group (mean difference (MD) -1.60 days, 95% CI -2.49 to -0.71; 68 participants). The other study found no evidence of a difference between the groups.Tranexamic acid versus other haemostatic agentsWhen we pooled the data from three studies the proportion of patients whose bleeding stopped within 10 minutes was significantly higher in the topical tranexamic acid group compared to the group receiving another haemostatic agent (70% versus 30%: RR 2.35, 95% CI 1.90 to 2.92; 460 participants) (moderate-quality evidence).Adverse effects across all studiesFive studies recorded 'adverse effects' in a general way. None found any difference between the groups in the occurrence of minor adverse effects (e.g. mild nausea and diarrhoea, 'bad taste' of gel). In one study a patient developed a superficial thrombophlebitis of both legs following discharge, however it is not reported in which group this occurred. No "other serious adverse effect" was reported in any study.
Authors' conclusions: We found moderate-quality evidence that there is probably a reduction in the risk of re-bleeding with the use of either oral or topical tranexamic acid in addition to usual care in adult patients with epistaxis, compared to placebo with usual care. However, the quality of evidence relating solely to topical tranexamic acid was low (one study only), so we are uncertain whether or not topical tranexamic acid is effective in stopping bleeding in the 10-day period after a single application. We found moderate-quality evidence that topical tranexamic acid is probably better than other topical agents in stopping bleeding in the first 10 minutes.There have been only three RCTs on this subject since 1995. Since then there have been significant changes in nasal cauterisation and packing techniques (for example, techniques including nasal endoscopy and more invasive approaches such as endoscopic sphenopalatine artery ligation). New trials would inform us about the effectiveness of tranexamic acid in light of these developments.
Conflict of interest statement
Jonathan Joseph: none known. Pablo Martinez‐Devesa: none known. Jenny Bellorini: Jenny Bellorini is Managing Editor of Cochrane ENT, but had no role in the editorial sign‐off process for this review. Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial sign‐off process for this review.
Figures







Update of
- doi: 10.1002/14651858.CD004328.pub2
Similar articles
-
Tranexamic acid for the reduction of bleeding during functional endoscopic sinus surgery.Cochrane Database Syst Rev. 2023 Feb 21;2(2):CD012843. doi: 10.1002/14651858.CD012843.pub2. Cochrane Database Syst Rev. 2023. PMID: 36808096 Free PMC article. Review.
-
Tranexamic acid for preventing postpartum haemorrhage after vaginal birth.Cochrane Database Syst Rev. 2025 Jan 15;1(1):CD007872. doi: 10.1002/14651858.CD007872.pub4. Cochrane Database Syst Rev. 2025. PMID: 39812173 Free PMC article.
-
Progestogen-releasing intrauterine systems for heavy menstrual bleeding.Cochrane Database Syst Rev. 2020 Jun 12;6(6):CD002126. doi: 10.1002/14651858.CD002126.pub4. Cochrane Database Syst Rev. 2020. PMID: 32529637 Free PMC article.
-
Interventions for chronic palmoplantar pustulosis.Cochrane Database Syst Rev. 2020 Jan 20;1(1):CD011628. doi: 10.1002/14651858.CD011628.pub2. Cochrane Database Syst Rev. 2020. PMID: 31958161 Free PMC article.
-
Oral H1 antihistamines as 'add-on' therapy to topical treatment for eczema.Cochrane Database Syst Rev. 2019 Jan 22;1(1):CD012167. doi: 10.1002/14651858.CD012167.pub2. Cochrane Database Syst Rev. 2019. PMID: 30666626 Free PMC article.
Cited by
-
Epistaxis Treatment Options: Literature Review.Indian J Otolaryngol Head Neck Surg. 2023 Sep;75(3):2235-2244. doi: 10.1007/s12070-023-03824-z. Epub 2023 May 8. Indian J Otolaryngol Head Neck Surg. 2023. PMID: 37636777 Free PMC article.
-
Efficacy and Safety of Tranexamic Acid in Bimaxillary Orthognathic Surgery.Plast Surg (Oakv). 2020 May;28(2):94-104. doi: 10.1177/2292550320925897. Epub 2020 May 21. Plast Surg (Oakv). 2020. PMID: 32596184 Free PMC article.
-
A comparative study of the local effect of tranexamic acid and phenylephrine on the amount of bleeding in rhinoplasty: A randomized clinical trial.Caspian J Intern Med. 2024 Sep 7;15(4):690-696. doi: 10.22088/cjim.154.690. eCollection 2024 Fall. Caspian J Intern Med. 2024. PMID: 39359446 Free PMC article.
-
Effects of tranexamic acid on human nasal ciliary beat frequency.Eur Arch Otorhinolaryngol. 2021 Sep;278(9):3351-3356. doi: 10.1007/s00405-020-06602-7. Epub 2021 Feb 4. Eur Arch Otorhinolaryngol. 2021. PMID: 33538874 Free PMC article.
-
Prospective review of 188 cases of epistaxis presenting to the emergency department: Etiology and outcome.J Family Med Prim Care. 2023 Nov;12(11):2721-2726. doi: 10.4103/jfmpc.jfmpc_889_23. Epub 2023 Nov 21. J Family Med Prim Care. 2023. PMID: 38186796 Free PMC article.
References
References to studies included in this review
Atabaki 2017 {published data only}
-
- Atabaki P, Samarei R, Aribi MS, Soheili A, Mehryar HR. A comparative study on the effect of topical phenylephrine with topical tranexamic acid in management of epistaxis. Journal of the Urmia Nursing and Midwifery Faculty 2017;15(7):488‐96.
Petruson 1974 {published data only}
-
- Petruson B. A double blind study to evaluate the effect on epistaxis with oral administration of the antifibrinolytic drug tranexamic acid (Cyklokapron®). Acta Otolaryngologica 1974;77(Suppl 317):57‐61.
Tibbelin 1995 {published data only}
-
- Tibbelin A, Aust R, Bende M, Holgersson M, Petruson B, Rundcrantz H, et al. Effect of local tranexamic acid gel in the treatment of epistaxis. ORL; Journal for Oto‐rhino‐laryngology and its Related Specialties 1995;57(4):207‐9. - PubMed
White 1988 {published data only}
-
- White A, O'Reilly BF. Oral tranexamic acid in the management of epistaxis. Clinical Otolaryngology and Allied Sciences 1998;13(1):11‐6. - PubMed
Zahed 2013 {published data only}
-
- Saeedi M, Arasi S. Effectiveness of local tranexamic acid on epistaxis control. Iranian Registry of Clinical Trials April 2012.
-
- Zahed R, Moharamzadeh P, Alizadeh Arasi S, Ghasemi A, Saeedi M. A new and rapid method for epistaxis treatment using injectable form of tranexamic acid topically: a randomized controlled trial. American Journal of Emergency Medicine 2013;31(9):1389‐92. - PubMed
Zahed 2018 {published data only}
-
- Zahed R, Mousavi Jazayeri M H, Naderi A, Naderpour Z, Saeedi M. Topical tranexamic acid compared with anterior nasal packing for treatment of epistaxis in patients taking antiplatelet drugs: randomized controlled trial. Academic Emergency Medicine 2018;25(3):261‐6. - PubMed
References to studies excluded from this review
Alimian 2011 {published data only}
-
- Alimian M, Mohseni M. The effect of intravenous tranexamic acid on blood loss and surgical field quality during endoscopic sinus surgery: a placebo‐controlled clinical trial. Journal of Clinical Anesthesia 2011;23:611‐5. - PubMed
ATERO 2014 {published data only}
-
- Gaillard S, Dupuis‐Girod S, Boutitie F, Rivière S, Morinière S, Hatron PY, et al. ATERO Study Group. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: a European cross‐over controlled trial in a rare disease. Journal of Thrombosis and Haemostasis 2014;12(9):1494‐502. - PubMed
Athanasiadis 2007 {published data only}
-
- Athanasiadis T, Beule A, Wormald P. Effects of topical antifibrinolytics in endoscopic sinus surgery: a pilot randomised controlled trial. American Journal of Rhinology 2007;21(6):737‐42. - PubMed
Baradaranfar 2017 {published data only}
Beikaei 2015 {published data only}
-
- Beikaei M, Ghazipour A, Derakhshande V, Saki N, Nikakhlagh S. Evaluating the effect of intravenous tranexamic acid on intraoperative bleeding during elective rhinoplasty surgery. Biomedical and Pharmacology Journal 2015;8SE:753‐9.
Chhapola 2011 {published data only}
-
- Chhapola S, Matta I. Short‐term use of tranexamic acid to reduce blood loss in endoscopic nasal surgeries. Clinical Rhinology 2011;4:79‐81.
Eftekharian 2016 {published data only}
-
- Eftekharian HR, Rajabzadeh Z. The efficacy of preoperative oral tranexamic acid on intraoperative bleeding during rhinoplasty. Journal of Craniofacial Surgery 2016;27(1):97‐100. - PubMed
Fernandez‐L 2007 {published data only}
-
- Fernandez‐L A, Garrido‐Martin EM, Sanz‐Rodriguez F, Ramirez J‐R, Morales‐Angulo C, Zarrabeita R, et al. Therapeutic action of tranexamic acid in hereditary haemorrhagic telangiectasia (HHT): regulation of ALK‐a/endoglin pathway in endothelial cells. Thrombosis Haemostasis 2007;97(2):254‐62. - PubMed
Geisthoff 2014 {published data only}
-
- Geisthoff UW, Seyfert U, Konig J, Kubler M, Bieg B, Plinkert PK. Hereditary hemorrhagic telangiectasia and tranexamic acid: a double‐blind placebo‐controlled crossover study. European Archive of Otorhinolaryngology 2004;261(1):41.
Ghavimi 2017 {published data only}
-
- Ghavimi MA, Taheri Talesh K, Ghoreishizadeh A, Chavoshzadeh MA, Zarandi A. Efficacy of tranexamic acid on side effects of rhinoplasty: a randomized double‐blind study. Journal of cranio‐maxillofacial surgery. Journal of Cranio‐maxillo‐facial Surgery 2017;45(6):897‐902. - PubMed
Gossage 2015 {published data only}
-
- Gossage JR, Whitehead KJ, Sautter NB, McWilliams JP, Chakinala M, Merlo C, et al. North American study of epistaxis in HHT (nose trial). Angiogenesis 2015;18(4):541‐2.
IRCT2014122520434N1 {published data only}
-
- IRCT2014122520434N1. Comparison of the effect of tranexamic acid and dexmedetomidine on intra‐operative bleeding in patients candidate for rhinoplasty. https://en.irct.ir/trial/18125 (first received 16 July 2015). [IRCT2014122520434N1]
IRCT201509088872N9 {published data only}
-
- IRCT201509088872N9. Effectiveness of local tranexamic acid on epistaxis control in patients with antiplateletes therapy (ASA, Plavix). http://www.irct.ir/ (first received 25 September 2015).
Jabalameli 2006 {published data only}
-
- Jabalameli M, Zakeri K. Evaluation of topical tranexamic acid on intraoperative bleeding in endoscopic sinus surgery. Iranian Journal of Medical Science 2006;31(4):221‐3.
Keiani Motlagh 2003 {published data only}
-
- Keiani Motlagh K, Loeb I, Legrand W, Daelemans P, Reck J. Prevention of postoperative bleeding in patients taking oral anticoagulants. Effects of tranexamic acid [Prevention des saignements postoperatoires chez des patients sous anticoagulants oraux; effets de l'acide tranexamique]. Revue de Stomatologie et de Chirurgie Maxillo‐Faciale 2003;104(2):77‐9. - PubMed
Kulkarni 2018 {published data only}
-
- Kulkarni VR. A comparative study of tranexamic acid and ehamsylate for control of blood loss in functional endoscopic sinus surgery. Paripex ‐ Indian Journal of Research 2018;7(6):58‐63.
Mehdizadeh 2018 {published data only}
-
- Mehdizadeh M, Ghassemi A, Khakzad M, Mir M, Nekoohesh L, Moghadamnia A, et al. Comparison of the effect of dexamethasone and tranexamic acid, separately or in combination on post‐rhinoplasty edema and ecchymosis. Aesthetic Plastic Surgery 2018;42(1):246‐52. - PubMed
NOSE 2012 {published data only}
-
- Gossage J. North American study of epistaxis in HHT. http://clinicaltrials.gov/ct2/show/NCT01408030 (accessed 6 August 2012). [NCT01408030]
Sabba 2001 {published data only}
-
- Sabba C, Gallitelli M, Palasciano G. Efficacy of unusually high doses of tranexamic acid for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. New England Journal of Medicine 2001;345(12):926. - PubMed
Whitehead 2016 {published data only}
-
- Whitehead KJ, Sautter NB, McWilliams JP, Chakinala MM, Merlo CA, Johnson MH, et al. Effect of topical intranasal therapy on epistaxis frequency in patients with hereditary hemorrhagic telangiectasia: a randomized clinical trial. JAMA 2016;316(9):943‐51. - PubMed
Yaniv 2006 {published data only}
-
- Yaniv E, Shvero J, Hadar T. Hemostatic effect of tranexamic acid in elective surgery. American Journal of Rhinology 2006;20(2):227‐9. - PubMed
References to ongoing studies
ISRCTN34153772 {published data only}
-
- ISRCTN34153772. Novel use of TXA to reduce the need for nasal packing in epistaxis. http://www.isrctn.com/ISRCTN34153772 (first received 1 June 2017).
NCT02930941 {published data only}
-
- NCT02930941. Topical intranasal tranexamic acid for epistaxis in the emergency department. https://clinicaltrials.gov/ct2/show/NCT02930941 (first received 12 October 2016).
NCT03360045 {published data only}
-
- NCT03360045. The evaluation of effectiveness of nasal compression with tranexamic acid compared to simple nasal compression and Merocel packing. https://clinicaltrials.gov/ct2/show/NCT03360045 (first received 2 December 2017). [NCT03360045]
Additional references
BRS 2017
-
- INTEGRATE (The National ENT Trainee Research Network), National ENT Trainee Research Network. The British Rhinological Society multidisciplinary consensus recommendations on the hospital management of epistaxis. Journal of Laryngology and Otology 2017;131(12):1142‐56. - PubMed
Dunn 1999
-
- Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs 1999;57(6):1005‐32. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kamhieh 2016
-
- Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clinical Otolaryngology 2016;41(6):771‐6. - PubMed
Ker 2012
McGarry 2008
-
- McGarry GW. Epistaxis. In: Gleeson M, Browning GG, Burton MJ, Clarke R, Hibbert J, Jones NS, et al. editor(s). Scott‐Brown's Otolaryngology, Head and Neck Surgery. 7th Edition. Vol. 2, Chapter 126, London: Edward Arnold, 2008:1596‐606.
Nishihara 2015
-
- Nishihara S, Hamada M. Does tranexamic acid alter the risk of thromboembolism after total hip arthroplasty in the absence of routine chemical thromboprophylaxis?. The Bone & Joint Journal 2015;97‐B(4):458‐62. - PubMed
Ravesloot 2017
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robb 2014
-
- Robb PJ. Tranexamic acid ‐ a useful drug in ENT surgery?. Journal of Laryngology and Otology 2014;128:574‐9. - PubMed
Roberts 2013
-
- Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH‐2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technology Assessment (Winchester, England) 2013;17(10):1‐79. - PMC - PubMed
Shaheen 1967
-
- Shaheen OH. Epistaxis in the Middle Aged and Elderly [Thesis]. London: University of London, 1967.
Sharma 2014
-
- Sharma V, Katznelson R, Jerath A, Garrido‐Olivares L, Carroll J, Rao V, et al. The association between tranexamic acid and convulsive seizures after cardiac surgery: a multivariate analysis in 11 529 patients. Anaesthesia 2014;69(2):124‐30. - PubMed
Stell 1977
-
- Stell PM. Epistaxis. Clinical Otolaryngology 1977;2:263‐73. - PubMed
UK Epistaxis Audit 2017
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

