Failure to reabsorb the primary cilium induces cellular senescence
- PMID: 30596512
- PMCID: PMC6436649
- DOI: 10.1096/fj.201801382R
Failure to reabsorb the primary cilium induces cellular senescence
Abstract
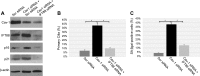
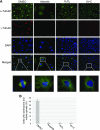
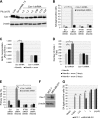
Aurora kinase A (AURKA) is necessary for proper primary cilium disassembly before mitosis. We found that depletion of caveolin-1 expression promotes primary cilia formation through the proteasomal-dependent degradation of aurora kinase A and induces premature senescence in human fibroblasts. Down-regulation of intraflagellar transport-88, a protein essential for ciliogenesis, inhibits premature senescence induced by the depletion of caveolin-1. In support of these findings, we showed that alisertib, a pharmacological inhibitor of AURKA, causes primary cilia formation and cellular senescence by irreversibly arresting cell growth. Suppression of primary cilia formation limits cellular senescence induced by alisertib. The primary cilium must be disassembled to free its centriole to form the centrosome, a necessary structure for mitotic spindle assembly and cell division. We showed that the use of the centriole to form primary cilia blocks centrosome formation and mitotic spindle assembly and prevents the completion of mitosis in cells in which cellular senescence is caused by the inhibition of AURKA. We also found that AURKA is down-regulated and primary cilia formation is enhanced when cellular senescence is promoted by other senescence-inducing stimuli, such as oxidative stress and UV light. Thus, we propose that impaired AURKA function induces premature senescence by preventing reabsorption of the primary cilium, which inhibits centrosome and mitotic spindle formation and consequently prevents the completion of mitosis. Our study causally links the inability of the cell to disassemble the primary cilium, a microtubule-based cellular organelle, to the development of premature senescence, a functionally and pathologically relevant cellular state.-Jeffries, E. P., Di Filippo, M., Galbiati, F. Failure to reabsorb the primary cilium induces cellular senescence.
Keywords: alisertib; aurora kinase A; caveolae; caveolin-1; mitotic spindle.
Conflict of interest statement
This work was supported by Grant R01-CA205165 from the U.S. National Institutes of Health (NIH) National Cancer Institute (to F.G.), and Grant R01-HL124747 from the NIH National Heart, Lung, and Blood Institute (to F.G.). The authors declare no conflicts of interest.
Figures












Similar articles
-
Primary cilium: an elaborate structure that blocks cell division?Gene. 2014 Sep 1;547(2):175-85. doi: 10.1016/j.gene.2014.06.050. Epub 2014 Jun 24. Gene. 2014. PMID: 24971504 Review.
-
Therapeutic potential of mitotic interaction between the nucleoporin Tpr and aurora kinase A.Cell Cycle. 2015;14(9):1447-58. doi: 10.1080/15384101.2015.1021518. Cell Cycle. 2015. PMID: 25789545 Free PMC article.
-
Mechanisms for nonmitotic activation of Aurora-A at cilia.Biochem Soc Trans. 2017 Feb 8;45(1):37-49. doi: 10.1042/BST20160142. Biochem Soc Trans. 2017. PMID: 28202658 Free PMC article. Review.
-
Selective targeting of non-centrosomal AURKA functions through use of a targeted protein degradation tool.Commun Biol. 2021 May 28;4(1):640. doi: 10.1038/s42003-021-02158-2. Commun Biol. 2021. PMID: 34050235 Free PMC article.
-
INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability.J Cell Sci. 2015 Jan 15;128(2):364-72. doi: 10.1242/jcs.161323. Epub 2014 Nov 13. J Cell Sci. 2015. PMID: 25395580 Free PMC article.
Cited by
-
Primary cilia in Parkinson's disease: summative roles in signaling pathways, genes, defective mitochondrial function, and substantia nigra dopaminergic neurons.Front Aging Neurosci. 2024 Sep 18;16:1451655. doi: 10.3389/fnagi.2024.1451655. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39364348 Free PMC article. Review.
-
The Primary Cilium as a Therapeutic Target in Ocular Diseases.Front Pharmacol. 2020 Jun 26;11:977. doi: 10.3389/fphar.2020.00977. eCollection 2020. Front Pharmacol. 2020. PMID: 32676032 Free PMC article. Review.
-
A stress-induced cilium-to-PML-NB route drives senescence initiation.Nat Commun. 2023 Apr 3;14(1):1840. doi: 10.1038/s41467-023-37362-7. Nat Commun. 2023. PMID: 37019904 Free PMC article.
-
Dysregulation of Caveolin-1 Phosphorylation and Nuclear Translocation Is Associated with Senescence Onset.Cells. 2021 Oct 28;10(11):2939. doi: 10.3390/cells10112939. Cells. 2021. PMID: 34831162 Free PMC article.
-
Structure, function, and research progress of primary cilia in reproductive physiology and reproductive diseases.Front Cell Dev Biol. 2024 Jun 3;12:1418928. doi: 10.3389/fcell.2024.1418928. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38887518 Free PMC article. Review.
References
-
- Ishikawa H., Marshall W. F. (2011) Ciliogenesis: building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 12, 222–234 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

