Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes†
- PMID: 30596885
- PMCID: PMC6497524
- DOI: 10.1093/biolre/ioy264
Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes†
Abstract
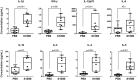
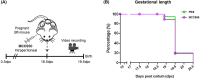
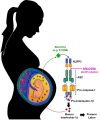
Sterile intra-amniotic inflammation is commonly observed in patients with spontaneous preterm labor, a syndrome that commonly precedes preterm birth, the leading cause of perinatal morbidity and mortality worldwide. However, the mechanisms leading to sterile intra-amniotic inflammation are poorly understood and no treatment exists for this clinical condition. Herein, we investigated whether the alarmin S100B could induce sterile intra-amniotic inflammation by activating the NLRP3 inflammasome, and whether the inhibition of this pathway could prevent preterm labor/birth and adverse neonatal outcomes. We found that the ultrasound-guided intra-amniotic administration of S100B induced a 50% rate of preterm labor/birth and a high rate of neonatal mortality (59.7%) without altering the fetal and placental weights. Using a multiplex cytokine array and immunoblotting, we reported that S100B caused a proinflammatory response in the amniotic cavity and induced the activation of the NLRP3 inflammasome in the fetal membranes, indicated by the upregulation of the NLRP3 protein and increased release of active caspase-1 and mature IL-1β. Inhibition of the NLRP3 inflammasome via the specific inhibitor MCC950 prevented preterm labor/birth by 35.7% and reduced neonatal mortality by 26.7%. Yet, inhibition of the NLRP3 inflammasome at term did not drastically obstruct the physiological process of parturition. In conclusion, the data presented herein indicate that the alarmin S100B can induce sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes by activating the NLRP3 inflammasome, which can be prevented by inhibiting such a pathway. These findings provide evidence that sterile intra-amniotic inflammation could be treated by targeting the NLRP3 inflammasome.
Keywords: S100B; acute chorioamnionitis; alarmins; amniotic fluid; caspase-1; cytokines; damage-associated molecular patterns; danger signals; funisitis; inhibitor; interleukin-1β; mice.
© The Author(s) 2018. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures




Similar articles
-
A key role for NLRP3 signaling in preterm labor and birth driven by the alarmin S100B.Transl Res. 2023 Sep;259:46-61. doi: 10.1016/j.trsl.2023.04.004. Epub 2023 Apr 28. Transl Res. 2023. PMID: 37121539 Free PMC article.
-
Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome†.Biol Reprod. 2019 May 1;100(5):1290-1305. doi: 10.1093/biolre/ioy261. Biol Reprod. 2019. PMID: 30590393 Free PMC article.
-
The alarmin interleukin-1α causes preterm birth through the NLRP3 inflammasome.Mol Hum Reprod. 2020 Sep 1;26(9):712-726. doi: 10.1093/molehr/gaaa054. Mol Hum Reprod. 2020. PMID: 32647859 Free PMC article.
-
The immunobiology of preterm labor and birth: intra-amniotic inflammation or breakdown of maternal-fetal homeostasis.Reproduction. 2022 Jun 20;164(2):R11-R45. doi: 10.1530/REP-22-0046. Reproduction. 2022. PMID: 35559791 Free PMC article. Review.
-
Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors.Cell Death Dis. 2019 Feb 12;10(2):128. doi: 10.1038/s41419-019-1413-8. Cell Death Dis. 2019. PMID: 30755589 Free PMC article. Review.
Cited by
-
Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone.J Immunol. 2019 May 1;202(9):2585-2608. doi: 10.4049/jimmunol.1801350. Epub 2019 Mar 27. J Immunol. 2019. PMID: 30918041 Free PMC article. Clinical Trial.
-
The role of intraamniotic inflammation in threatened midtrimester miscarriage.Am J Obstet Gynecol. 2022 Dec;227(6):895.e1-895.e13. doi: 10.1016/j.ajog.2022.07.007. Epub 2022 Jul 16. Am J Obstet Gynecol. 2022. PMID: 35843271 Free PMC article.
-
Screening of Chorioamnionitis Using Volatile Organic Compound Detection in Exhaled Breath: A Pre-clinical Proof of Concept Study.Front Pediatr. 2021 May 26;9:617906. doi: 10.3389/fped.2021.617906. eCollection 2021. Front Pediatr. 2021. PMID: 34123958 Free PMC article.
-
Frontiers in the Etiology and Treatment of Preterm Premature Rupture of Membrane: From Molecular Mechanisms to Innovative Therapeutic Strategies.Reprod Sci. 2024 Apr;31(4):917-931. doi: 10.1007/s43032-023-01411-9. Epub 2023 Nov 21. Reprod Sci. 2024. PMID: 37989803 Review.
-
The alarmin S100A12 causes sterile inflammation of the human chorioamniotic membranes as well as preterm birth and neonatal mortality in mice†.Biol Reprod. 2021 Dec 20;105(6):1494-1509. doi: 10.1093/biolre/ioab188. Biol Reprod. 2021. PMID: 34632484 Free PMC article.
References
-
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet North Am Ed 2015; 385:430–440. - PubMed
-
- Murphy SL, Mathews TJ, Martin JA, Minkovitz CS, Strobino DM. Annual summary of vital statistics: 2013–2014. Pediatrics 2017; 139:e20163239. - PubMed
-
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014; 71:330–358. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

