Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity
- PMID: 30596910
- PMCID: PMC6308314
- DOI: 10.1093/brain/awy313
Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity
Abstract
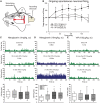
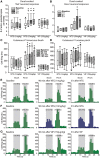
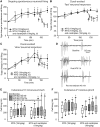
Cranial allodynia associated with spontaneous migraine is reported as either responsive to triptan treatment or to be predictive of lack of triptan efficacy. These conflicting results suggest that a single mechanism mediating the underlying neurophysiology of migraine symptoms is unlikely. The lack of a translational approach to study cranial allodynia reported in migraine patients is a limitation in dissecting potential mechanisms. Our objective was to study triptan-responsive cranial allodynia in migraine patients, and to develop an approach to studying its neural basis in the laboratory. Using nitroglycerine to trigger migraine attacks, we investigated whether cranial allodynia could be triggered experimentally, observing its response to treatment. Preclinically, we examined the cephalic response properties of central trigeminocervical neurons using extracellular recording techniques, determining changes to ongoing firing and somatosensory cranial-evoked sensitivity, in response to nitroglycerine followed by triptan treatment. Cranial allodynia was triggered alongside migraine-like headache in nearly half of subjects. Those who reported cranial allodynia accompanying their spontaneous migraine attacks were significantly more likely to have symptoms triggered than those that did not. Patients responded to treatment with aspirin or sumatriptan. Preclinically, nitroglycerine caused an increase in ongoing firing and hypersensitivity to intracranial-dural and extracranial-cutaneous (noxious and innocuous) somatosensory stimulation, reflecting signatures of central sensitization potentially mediating throbbing headache and cranial allodynia. These responses were aborted by a triptan. These data suggest that nitroglycerine can be used as an effective and reliable method to trigger cranial allodynia in subjects during evoked migraine, and the symptom is responsive to abortive triptan treatments. Preclinically, nitroglycerine activates the underlying neural mechanism of cephalic migraine symptoms, central sensitization, also predicting the clinical outcome to triptans. This supports a biological rationale that several mechanisms can mediate the underlying neurophysiology of migraine symptoms, with nitrergic-induced changes reflecting one that is relevant to spontaneous migraine in many migraineurs, whose symptoms of cranial allodynia are responsive to triptan treatment. This approach translates directly to responses in animals and is therefore a relevant platform to study migraine pathophysiology, and for use in migraine drug discovery.
Figures

Comment in
-
Dissecting out migraine complexity through comprehensive analysis of allodynia.Brain. 2019 Jan 1;142(1):5-8. doi: 10.1093/brain/awy315. Brain. 2019. PMID: 30596909 No abstract available.
References
-
- Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain 2004; 110: 675–80. - PubMed
-
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005; 128 (Pt 4): 932–9. - PubMed
-
- Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci Transl Med 2015; 7: 308ra157. - PubMed
-
- Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 2011; 12: 570–84. - PubMed
-
- Akerman S, Holland PR, Lasalandra MP, Goadsby PJ. Inhibition of trigeminovascular dural nociceptive afferents by Ca(2+)-activated K(+) (MaxiK/BK(Ca)) channel opening. Pain 2010; 151: 128–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

