Synthetic biology for bioengineering virus-like particle vaccines
- PMID: 30597533
- PMCID: PMC7161758
- DOI: 10.1002/bit.26890
Synthetic biology for bioengineering virus-like particle vaccines
Abstract
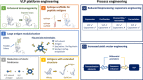
Vaccination is the most effective method of disease prevention and control. Many viruses and bacteria that once caused catastrophic pandemics (e.g., smallpox, poliomyelitis, measles, and diphtheria) are either eradicated or effectively controlled through routine vaccination programs. Nonetheless, vaccine manufacturing remains incredibly challenging. Viruses exhibiting high antigenic diversity and high mutation rates cannot be fairly contested using traditional vaccine production methods and complexities surrounding the manufacturing processes, which impose significant limitations. Virus-like particles (VLPs) are recombinantly produced viral structures that exhibit immunoprotective traits of native viruses but are noninfectious. Several VLPs that compositionally match a given natural virus have been developed and licensed as vaccines. Expansively, a plethora of studies now confirms that VLPs can be designed to safely present heterologous antigens from a variety of pathogens unrelated to the chosen carrier VLPs. Owing to this design versatility, VLPs offer technological opportunities to modernize vaccine supply and disease response through rational bioengineering. These opportunities are greatly enhanced with the application of synthetic biology, the redesign and construction of novel biological entities. This review outlines how synthetic biology is currently applied to engineer VLP functions and manufacturing process. Current and developing technologies for the identification of novel target-specific antigens and their usefulness for rational engineering of VLP functions (e.g., presentation of structurally diverse antigens, enhanced antigen immunogenicity, and improved vaccine stability) are described. When applied to manufacturing processes, synthetic biology approaches can also overcome specific challenges in VLP vaccine production. Finally, we address several challenges and benefits associated with the translation of VLP vaccine development into the industry.
Keywords: capsomere; computational; omics technologies; synthetic biology; vaccine; virus-like particle.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
The University of Queensland (UQ) filed patents on the use of MuPyV as a vaccine platform. L. H. L. L. and A. P. J. M. contributed to those patents and, through their employment with UQ, hold an indirect interest in this intellectual property.
Figures

References
-
- Anggraeni, M. R. , Connors, N. K. , Wu, Y. , Chuan, Y. P. , Lua, L. H. L. , & Middelberg, A. P. J. (2013). Sensitivity of immune response quality to influenza helix 190 antigen structure displayed on a modular virus‐like particle. Vaccine, 31(40), 4428–4435. - PubMed
-
- Arora, U. , Tyagi, P. , Swaminathan, S. , & Khanna, N. (2013). Virus‐like particles displaying envelope domain III of dengue virus type 2 induce virus‐specific antibody response in mice. Vaccine, 31(6), 873–878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

