PF-04447943, a Phosphodiesterase 9A Inhibitor, in Stable Sickle Cell Disease Patients: A Phase Ib Randomized, Placebo-Controlled Study
- PMID: 30597771
- PMCID: PMC6440678
- DOI: 10.1111/cts.12604
PF-04447943, a Phosphodiesterase 9A Inhibitor, in Stable Sickle Cell Disease Patients: A Phase Ib Randomized, Placebo-Controlled Study
Abstract
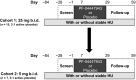
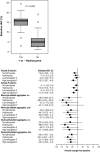
This phase Ib study randomized patients with stable sickle cell disease (SCD) aged 18-65 years to twice-daily PF-04447943 (a phosphodiesterase 9A inhibitor; 5 or 25 mg) or placebo, with/without hydroxyurea coadministration, for up to 29 days. Blood samples were collected at baseline and various posttreatment time points for assessments of PF-04447943 pharmacokinetics (PKs)/pharmacodynamics (PDs). Change from baseline in potential SCD-related biomarkers was evaluated. Of 30 patients, 15 received hydroxyurea and 28 completed the study. PF-04447943, with/without hydroxyurea, was generally well tolerated, with no treatment-related serious adverse events. Plasma PF-04447943 exposure was dose proportional. Twice-daily PF-04447943 25 mg significantly reduced the number and size of circulating monocyte-platelet and neutrophil-platelet aggregates and levels of circulating soluble E-selectin at day 29 vs. baseline (adjusted P < 0.15). PF-04447943 demonstrated PK/PD effects suggestive of inhibiting pathways that may contribute to vaso-occlusion. This study also provides guidance regarding biomarkers for future SCD studies.
Trial registration: ClinicalTrials.gov NCT02114203.
© 2018 Pfizer Inc. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
R.J.C., D.B., D.R., D.D.P., and N.C. are employees of Pfizer Inc and may own stock/options in the company. B.T. was an employee of Pfizer, Inc at the time of this study. J.H. is a consultant for Bluebird Bio, GBT, and Pfizer, and a member of the Speakers’ Bureau for Addmedica, Novartis, and Terumo BCT. A.D.M. has served as a consultant for Instrumentation Laboratory; receives research funding from Baxalta, Eisai, GLSynthesis, Ionis, Ironwood, Pfizer, and Sysmex; and is a member of advisory committees for AstraZeneca and Janssen. A.L.F. has received research funding from Baxalta, Eisai, GLSynthesis, Ionis, Ironwood, Pfizer, and Sysmex.
Figures


References
-
- Piel, F.B. , Steinberg, M.H. & Rees, D.C. Sickle cell disease. N. Engl. J. Med. 376, 1561–1573 (2017). - PubMed
-
- Hassell, K.L. Population estimates of sickle cell disease in the U.S. Am. J. Prev. Med. 38, S512–S521 (2010). - PubMed
-
- Hoover, R. , Rubin, R. , Wise, G. & Warren, R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood 54, 872–876 (1979). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

