Pre-Existing Dengue Immunity Drives a DENV-Biased Plasmablast Response in ZIKV-Infected Patient
- PMID: 30597938
- PMCID: PMC6356269
- DOI: 10.3390/v11010019
Pre-Existing Dengue Immunity Drives a DENV-Biased Plasmablast Response in ZIKV-Infected Patient
Abstract
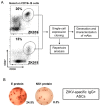
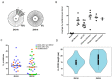
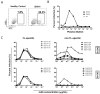
The re-emergence of Zika virus (ZIKV) in the western hemisphere has most significantly affected dengue virus (DENV) endemic regions. Due to the geographical overlap between these two closely related flaviviruses, numerous individuals who suffered ZIKV infection during recent outbreaks may have also previously been exposed to DENV. As such, the impact of pre-existing dengue immunity on immune responses to ZIKV has been an area of focused research and interest. To understand how B cell responses to a ZIKV infection may be modulated by prior dengue exposures, we compared and contrasted plasmablast repertoire and specificity between two ZIKV-infected individuals, one dengue-naïve (ZK018) and the other dengue-experienced (ZK016). In addition to examining serological responses, we generated 59 patient plasmablast-derived monoclonal antibodies (mAbs) to define the heterogeneity of the early B cell response to ZIKV. Both donors experienced robust ZIKV-induced plasmablast expansions early after infection, with comparable mutational frequencies in their antibody variable genes. However, notable differences were observed in plasmablast clonality and functional reactivity. Plasmablasts from the dengue-experienced donor ZK016 included cells with shared clonal origin, while ZK018 mAbs were entirely clonally unrelated. Both at the mAb and plasma level, ZK016 antibodies displayed extensive cross-reactivity to DENV1-4, and preferentially neutralized DENV compared to ZIKV. In contrast, the neutralization activity of ZK018 mAbs was primarily directed towards ZIKV, and fewer mAbs from this donor were cross-reactive, with the cross-reactive phenotype largely limited to fusion loop-specific mAbs. ZK016 antibodies caused greater enhancement of DENV2 infection of FcRγ-expressing cells overall compared to ZK018, with a striking difference at the plasma level. Taken together, these data strongly suggest that the breadth and protective capacity of the initial antibody responses after ZIKV infection may depend on the dengue immune status of the individual. These findings have implications for vaccine design, given the likelihood that future epidemics will involve both dengue-experienced and naïve populations.
Keywords: B-cell; DENV; ZIKV; antibodies; cross-reactivity; plasmablast.
Conflict of interest statement
N.R. has received funds from Merck, Pfizer and Sanofi Pasteur for research activities unrelated to this work. All authors declare no conflict of interest.
Figures
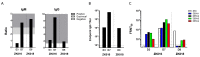


References
-
- Styczynski A.R., Malta J., Krow-Lucal E.R., Percio J., Nobrega M.E., Vargas A., Lanzieri T.M., Leite P.L., Staples J.E., Fischer M.X., et al. Increased rates of Guillain-Barre syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl. Trop. Dis. 2017;11:e0005869. doi: 10.1371/journal.pntd.0005869. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

