Profile of Phosphatidylserine Modifications under Nitroxidative Stress Conditions Using a Liquid Chromatography-Mass Spectrometry Based Approach
- PMID: 30597957
- PMCID: PMC6337642
- DOI: 10.3390/molecules24010107
Profile of Phosphatidylserine Modifications under Nitroxidative Stress Conditions Using a Liquid Chromatography-Mass Spectrometry Based Approach
Abstract
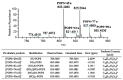
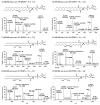
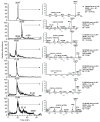
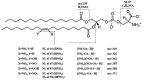
Nitrated lipids have been detected in vitro and in vivo, usually associated with a protective effect. While nitrated fatty acids have been widely studied, few studies reported the nitration and nitroxidation of the phospholipid classes phosphatidylcholine, and phosphatidylethanolamine. However, no information regarding nitrated and nitroxidized phosphatidylserine can be found in the literature. This work aims to identify and characterize the nitrated and nitroxidized derivatives of 1-palmitoyl-2-oleoyl-sn-3-glycero-phosphoserine (POPS), obtained after incubation with nitronium tetrafluoroborate, by liquid chromatography (LC) coupled to mass spectrometry (MS) and tandem MS (MS/MS). Several nitrated and nitroxidized products were identified, namely, nitro, nitroso, nitronitroso, and dinitro derivatives, as well as some nitroxidized species such as nitrosohydroxy, nitrohydroxy, and nitrohydroperoxy. The fragmentation pathways identified were structure-dependent and included the loss of HNO and HNO₂ for nitroso and nitro derivatives, respectively. Combined losses of PS polar head group plus HNO or HNO₂ and carboxylate anions of modified fatty acyl chain were also observed. The nitrated POPS also showed antiradical potential, demonstrated by the ability to scavenge the ABTS●+ and DPPH● radicals. Overall, this in vitro model of nitration based on LC-MS/MS provided additional insights into the nitrated and nitroxidized derivatives of PS and their fragmentation fingerprinting. This information is a valuable tool for targeted analysis of these modified PS in complex biological samples, to further explore the new clues on the antioxidant potential of nitrated POPS.
Keywords: LC-MS; lipidomic; nitration; nitroxidative stress; phosphatidylserine; tandem mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
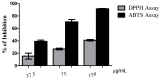
Similar articles
-
Analysis of Phosphatidylinositol Modifications by Reactive Nitrogen Species Using LC-MS: Coming to Grips with Their Nitroxidative Susceptibility.J Am Soc Mass Spectrom. 2023 Jul 5;34(7):1372-1382. doi: 10.1021/jasms.3c00057. Epub 2023 Jun 21. J Am Soc Mass Spectrom. 2023. PMID: 37343944 Free PMC article.
-
Characterization of phospholipid nitroxidation by LC-MS in biomimetic models and in H9c2 Myoblast using a lipidomic approach.Free Radic Biol Med. 2017 May;106:219-227. doi: 10.1016/j.freeradbiomed.2017.02.033. Epub 2017 Feb 17. Free Radic Biol Med. 2017. PMID: 28219782
-
Liquid chromatography/tandem mass spectrometry characterization of nitroso, nitrated and nitroxidized cardiolipin products.Free Radic Biol Med. 2019 Nov 20;144:183-191. doi: 10.1016/j.freeradbiomed.2019.05.009. Epub 2019 May 13. Free Radic Biol Med. 2019. PMID: 31095999
-
Understanding the nitrolipidome: From chemistry to mass spectrometry and biological significance of modified complex lipids.Prog Lipid Res. 2022 Jul;87:101176. doi: 10.1016/j.plipres.2022.101176. Epub 2022 May 27. Prog Lipid Res. 2022. PMID: 35636567 Review.
-
Oxidized and nitrated oleic acid in biological systems: analysis by GC-MS/MS and LC-MS/MS, and biological significance.Biochim Biophys Acta. 2011 Nov;1811(11):694-705. doi: 10.1016/j.bbalip.2011.06.015. Epub 2011 Jun 26. Biochim Biophys Acta. 2011. PMID: 21771665 Review.
Cited by
-
Formation of Oxidatively Modified Lipids as the Basis for a Cellular Epilipidome.Front Endocrinol (Lausanne). 2020 Dec 21;11:602771. doi: 10.3389/fendo.2020.602771. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33408694 Free PMC article. Review.
-
Advancing Target Identification of Nitrated Phospholipids in Biological Systems by HCD Specific Fragmentation Fingerprinting in Orbitrap Platforms.Molecules. 2020 May 1;25(9):2120. doi: 10.3390/molecules25092120. Molecules. 2020. PMID: 32369981 Free PMC article.
-
Analysis of Phosphatidylinositol Modifications by Reactive Nitrogen Species Using LC-MS: Coming to Grips with Their Nitroxidative Susceptibility.J Am Soc Mass Spectrom. 2023 Jul 5;34(7):1372-1382. doi: 10.1021/jasms.3c00057. Epub 2023 Jun 21. J Am Soc Mass Spectrom. 2023. PMID: 37343944 Free PMC article.
-
The Skin Epilipidome in Stress, Aging, and Inflammation.Front Endocrinol (Lausanne). 2021 Jan 21;11:607076. doi: 10.3389/fendo.2020.607076. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33551998 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

