Patched1-ArhGAP36-PKA-Inversin axis determines the ciliary translocation of Smoothened for Sonic Hedgehog pathway activation
- PMID: 30598432
- PMCID: PMC6338837
- DOI: 10.1073/pnas.1804042116
Patched1-ArhGAP36-PKA-Inversin axis determines the ciliary translocation of Smoothened for Sonic Hedgehog pathway activation
Abstract
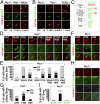
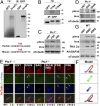
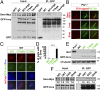
The Sonic Hedgehog (Shh) pathway conducts primarily in the primary cilium and plays important roles in cell proliferation, individual development, and tumorigenesis. Shh ligand binding with its ciliary membrane-localized transmembrane receptor Patched1 results in the removal of Patched1 from and the translocation of the transmembrane oncoprotein Smoothened into the cilium, leading to Shh signaling activation. However, how these processes are coupled remains unknown. Here, we show that the Patched1-ArhGAP36-PKA-Inversin axis determines the ciliary translocation of Smoothened. We find that Patched1 interacts with and stabilizes the PKA negative regulator ArhGAP36 to the centrosome. Activating the Shh pathway results in the removal of ArhGAP36 from the mother centriole and the centrosomal PKA accumulation. This PKA then phosphorylates Inversin and promotes its interaction with and the ciliary translocation of Smoothened. Knockdown of Inversin disrupts the ciliary translocation of Smoothened and Shh pathway activation. These findings reveal a regulatory molecular mechanism for the initial step of Shh pathway activation.
Keywords: Inversin; PKA; Patched1; Smoothened; Sonic Hedgehog pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Signaling in the primary cilium through the lens of the Hedgehog pathway.Wiley Interdiscip Rev Dev Biol. 2020 Nov;9(6):e377. doi: 10.1002/wdev.377. Epub 2020 Feb 21. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 32084300 Free PMC article. Review.
-
Macroautophagy supports Sonic Hedgehog signaling by promoting Patched1 degradation.Biochim Biophys Acta Mol Cell Res. 2021 Nov;1868(12):119124. doi: 10.1016/j.bbamcr.2021.119124. Epub 2021 Aug 20. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 34419491
-
Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA.Cell Signal. 2013 Nov;25(11):2222-30. doi: 10.1016/j.cellsig.2013.07.012. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872071 Free PMC article.
-
Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium.Proc Natl Acad Sci U S A. 2017 Dec 26;114(52):E11141-E11150. doi: 10.1073/pnas.1717891115. Epub 2017 Dec 11. Proc Natl Acad Sci U S A. 2017. PMID: 29229834 Free PMC article.
-
Endocytic Control of Cellular Signaling at the Primary Cilium.Trends Biochem Sci. 2016 Sep;41(9):784-797. doi: 10.1016/j.tibs.2016.06.002. Epub 2016 Jun 27. Trends Biochem Sci. 2016. PMID: 27364476 Review.
Cited by
-
Developmental and regenerative paradigms of cilia regulated hedgehog signaling.Semin Cell Dev Biol. 2021 Feb;110:89-103. doi: 10.1016/j.semcdb.2020.05.029. Epub 2020 Jun 12. Semin Cell Dev Biol. 2021. PMID: 32540122 Free PMC article. Review.
-
Critical roles of ARHGAP36 as a signal transduction mediator of Shh pathway in lateral motor columnar specification.Elife. 2019 Jul 15;8:e46683. doi: 10.7554/eLife.46683. Elife. 2019. PMID: 31305241 Free PMC article.
-
Enhancer hijacking at the ARHGAP36 locus is associated with connective tissue to bone transformation.Nat Commun. 2023 Apr 11;14(1):2034. doi: 10.1038/s41467-023-37585-8. Nat Commun. 2023. PMID: 37041138 Free PMC article.
-
Proteomic Profiling of Plasma-Derived Biomarkers in Patients with Bladder Cancer: A Step towards Clinical Translation.Life (Basel). 2021 Nov 25;11(12):1294. doi: 10.3390/life11121294. Life (Basel). 2021. PMID: 34947825 Free PMC article.
-
Signaling in the primary cilium through the lens of the Hedgehog pathway.Wiley Interdiscip Rev Dev Biol. 2020 Nov;9(6):e377. doi: 10.1002/wdev.377. Epub 2020 Feb 21. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 32084300 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

