Commensal Gut Bacteria Convert the Immunosuppressant Tacrolimus to Less Potent Metabolites
- PMID: 30598508
- PMCID: PMC6367689
- DOI: 10.1124/dmd.118.084772
Commensal Gut Bacteria Convert the Immunosuppressant Tacrolimus to Less Potent Metabolites
Abstract
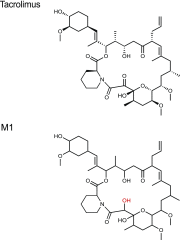
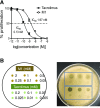
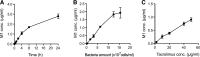
Tacrolimus exhibits low and variable drug exposure after oral dosing, but the contributing factors remain unclear. Based on our recent report showing a positive correlation between fecal abundance of Faecalibacterium prausnitzii and oral tacrolimus dose in kidney transplant patients, we tested whether F. prausnitzii and other gut abundant bacteria are capable of metabolizing tacrolimus. Incubation of F. prausnitzii with tacrolimus led to production of two compounds (the major one named M1), which was not observed upon tacrolimus incubation with hepatic microsomes. Isolation, purification, and structure elucidation using mass spectrometry and nuclear magnetic resonance spectroscopy indicated that M1 is a C-9 keto-reduction product of tacrolimus. Pharmacological activity testing using human peripheral blood mononuclear cells demonstrated that M1 is 15-fold less potent than tacrolimus as an immunosuppressant. Screening of 22 gut bacteria species revealed that most Clostridiales bacteria are extensive tacrolimus metabolizers. Tacrolimus conversion to M1 was verified in fresh stool samples from two healthy adults. M1 was also detected in the stool samples from kidney transplant recipients who had been taking tacrolimus orally. Together, this study presents gut bacteria metabolism as a previously unrecognized elimination route of tacrolimus, potentially contributing to the low and variable tacrolimus exposure after oral dosing.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. (1990) Basic local alignment search tool. J Mol Biol 215:403–410. - PubMed
-
- Dubbelboer IR, Pohanka A, Said R, Rosenborg S, Beck O. (2012) Quantification of tacrolimus and three demethylated metabolites in human whole blood using LC-ESI-MS/MS. Ther Drug Monit 34:134–142. - PubMed
-
- Edgar RC. (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. - PubMed
-
- Floren LC, Bekersky I, Benet LZ, Mekki Q, Dressler D, Lee JW, Roberts JP, Hebert MF. (1997) Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin Pharmacol Ther 62:41–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

