Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections
- PMID: 30598527
- PMCID: PMC6494982
- DOI: 10.1038/s41593-018-0301-3
Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections
Abstract
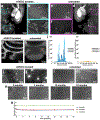
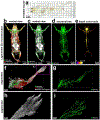
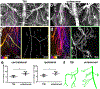
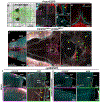
Analysis of entire transparent rodent bodies after clearing could provide holistic biological information in health and disease, but reliable imaging and quantification of fluorescent protein signals deep inside the tissues has remained a challenge. Here, we developed vDISCO, a pressure-driven, nanobody-based whole-body immunolabeling technology to enhance the signal of fluorescent proteins by up to two orders of magnitude. This allowed us to image and quantify subcellular details through bones, skin and highly autofluorescent tissues of intact transparent mice. For the first time, we visualized whole-body neuronal projections in adult mice. We assessed CNS trauma effects in the whole body and found degeneration of peripheral nerve terminals in the torso. Furthermore, vDISCO revealed short vascular connections between skull marrow and brain meninges, which were filled with immune cells upon stroke. Thus, our new approach enables unbiased comprehensive studies of the interactions between the nervous system and the rest of the body.
Figures


References
-
- Tuchin VV & Tuchin V Tissue optics: light scattering methods and instruments for medical diagnosis. Vol. 642 (SPIE press; Bellingham, 2007).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

