Patch-augmented rotator cuff surgery (PARCS) study-protocol for a feasibility study
- PMID: 30598834
- PMCID: PMC6302398
- DOI: 10.1186/s40814-018-0380-7
Patch-augmented rotator cuff surgery (PARCS) study-protocol for a feasibility study
Abstract
Background: A rotator cuff tear is a common disabling shoulder problem. Symptoms include pain, weakness, lack of shoulder mobility and sleep disturbance. Many patients require surgery to repair the tear; however, there is a high failure rate. There is a pressing need to improve the outcome of rotator cuff surgery and the use of patch augmentation to provide support to the healing process and improve patient outcomes holds new promise. Patches have been made using different materials (e.g. human/animal skin or intestine tissue, and completely synthetic materials) and processes (e.g. woven or a mesh). However, clinical evidence on their use is limited. The aim of the patch-augmented rotator cuff surgery (PARCS) feasibility study is to determine, using a mixed method approach, the design of a definitive randomised trial assessing the effectiveness and cost-effectiveness of a patch to augment surgical repair of the rotator cuff that is both acceptable to stakeholders and feasible.
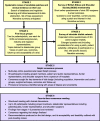
Methods: The objectives of this six-stage mixed methods feasibility study are to determine current practice, evidence and views about patch use; achieve consensus on the design of a randomised trial to evaluate patch-augmented rotator cuff surgery; and assess the acceptability and feasibility of the proposed design. The six stages will involve a systematic review of clinical evidence, two surveys of surgeons, focus groups and interviews with stakeholders, a Delphi study and a consensus meeting. The various stakeholders (including patients, surgeons, and representatives from industry, the NHS and regulatory bodies) will be involved across the six stages.
Discussion: The PARCS feasibility study will inform the feasibility and acceptability of a randomised trial of the effectiveness and cost-effectiveness of a patch-augmented rotator cuff surgery. Consensus opinion on the basic design of a randomised trial will be sought.
Trial registration: Not applicable.
Keywords: Dermal matrix; Feasibility study; Randomised trial; Rotator cuff tear; Shoulder surgery; Surgical mesh; Tissue scaffold.
Conflict of interest statement
This feasibility study was reviewed and approved to proceed by the University of Oxford Joint Research Office (JRO) study classification group (ref: PID13023). The JRO determined that the PARCS study falls outside of the definition of research that requires Health Research Authority (HRA) ethical approval and is therefore was not subject to the Department of Health’s Research Governance Framework for Health and Social Care (2005). It does not therefore require further assessment or approval prior to commencement. Informed Consent will be sought from participants as detailed in the main body of this paper.Not applicable.Andrew Carr has applied for a patent that will be considered as part of this work. We have carefully designed a robust research strategy that incorporates substantial independent input throughout to ensure no other individual, whether they be within or out the project team, can have undue influence on the process. Amar Rangan has received educational and research grants from DePuy Ltd. outside the scope of this work. The authors declare that they have no conflict of interest.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Patch augmentation surgery for rotator cuff repair: the PARCS mixed-methods feasibility study.Health Technol Assess. 2021 Feb;25(13):1-138. doi: 10.3310/hta25130. Health Technol Assess. 2021. PMID: 33646096 Free PMC article.
-
Findings from the patch augmented rotator cuff surgery (PARCS) feasibility study.Pilot Feasibility Stud. 2021 Aug 20;7(1):163. doi: 10.1186/s40814-021-00899-9. Pilot Feasibility Stud. 2021. PMID: 34416915 Free PMC article.
-
Systematic review of the surgical management of rotator cuff repair with an augmentative patch: a feasibility study protocol.Syst Rev. 2018 Nov 13;7(1):187. doi: 10.1186/s13643-018-0851-1. Syst Rev. 2018. PMID: 30424809 Free PMC article.
-
The use of a patch to augment rotator cuff surgery - A survey of UK shoulder and elbow surgeons.PLoS One. 2020 Apr 2;15(4):e0230235. doi: 10.1371/journal.pone.0230235. eCollection 2020. PLoS One. 2020. PMID: 32240199 Free PMC article.
-
Shoulder replacement surgery for osteoarthritis and rotator cuff tear arthropathy.Cochrane Database Syst Rev. 2020 Apr 21;4(4):CD012879. doi: 10.1002/14651858.CD012879.pub2. Cochrane Database Syst Rev. 2020. PMID: 32315453 Free PMC article.
Cited by
-
Patch augmentation surgery for rotator cuff repair: the PARCS mixed-methods feasibility study.Health Technol Assess. 2021 Feb;25(13):1-138. doi: 10.3310/hta25130. Health Technol Assess. 2021. PMID: 33646096 Free PMC article.
-
Use of implantable meshes for augmented rotator cuff repair: a systematic review and meta-analysis.BMJ Open. 2020 Dec 7;10(12):e039552. doi: 10.1136/bmjopen-2020-039552. BMJ Open. 2020. PMID: 33293307 Free PMC article.
-
Findings from the patch augmented rotator cuff surgery (PARCS) feasibility study.Pilot Feasibility Stud. 2021 Aug 20;7(1):163. doi: 10.1186/s40814-021-00899-9. Pilot Feasibility Stud. 2021. PMID: 34416915 Free PMC article.
-
Defining and measuring acceptability of surgical interventions: A scoping review.PLoS One. 2025 Jun 3;20(6):e0323738. doi: 10.1371/journal.pone.0323738. eCollection 2025. PLoS One. 2025. PMID: 40460189 Free PMC article.
-
Surgical Technique for Arthroscopic Rotator Cuff Augmentation With Human Acellular Dermal Matrix.Arthrosc Tech. 2021 Mar 8;10(4):e1025-e1032. doi: 10.1016/j.eats.2020.12.002. eCollection 2021 Apr. Arthrosc Tech. 2021. PMID: 33981546 Free PMC article.
References
-
- Goldgrub R, Cote P, Sutton D, Wong JJ, Yu H, Randhawa K, et al. The effectiveness of multimodal care for the management of soft tissue injuries of the shoulder: a systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) collaboration. J Manip Physiol Ther. 2016;39(2):121–139. doi: 10.1016/j.jmpt.2016.01.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources