Food-Grade Saponin Extract as an Emulsifier and Immunostimulant in Emulsion-Based Subunit Vaccine for Pigs
- PMID: 30599004
- PMCID: PMC6288570
- DOI: 10.1155/2018/8979838
Food-Grade Saponin Extract as an Emulsifier and Immunostimulant in Emulsion-Based Subunit Vaccine for Pigs
Abstract
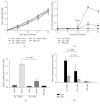
Subunit vaccines consisting of highly purified antigens require the presence of adjuvants to create effective and long-lasting protective immunity. Advances on adjuvant research include designing combination adjuvants which incorporate two or more adjuvants to enhance vaccine efficacy. Previously, an oil-in-water emulsion adjuvant (OW-14) composed of mineral oil and an inexpensive gum Arabic emulsifier has been reported demonstrating enhanced and robust immune responses when used as an adjuvant in swine subunit vaccines. This study presents a modified version of OW-14 prepared with food-grade Quillaja saponin extract (OWq). In new OWq emulsion, saponin extract served as an emulsifier for stabilization of emulsion droplets and as an immunoactive compound. The use of saponins allowed to reduce the required amount of emulsifier in the original OW-14. However, emulsion stabilized with saponins demonstrated extended physical stability even at elevated temperature (37°C). The two-dose vaccination with a classical swine fever virus (CSFV) glycoprotein E2-based vaccine formulated with OWq produced higher levels of E2-specific IgG and virus neutralizing antibodies in pigs in contrast with animals that received the vaccine adjuvanted with oil only. In addition, new OWq adjuvant was safe to use in the vaccination of pigs.
Figures


Similar articles
-
Enhanced protective immunity to CSFV E2 subunit vaccine by using IFN-γ as immunoadjuvant in weaning piglets.Vaccine. 2018 Nov 19;36(48):7353-7360. doi: 10.1016/j.vaccine.2018.10.030. Epub 2018 Oct 23. Vaccine. 2018. PMID: 30366801
-
Plant-made E2 glycoprotein single-dose vaccine protects pigs against classical swine fever.Plant Biotechnol J. 2019 Feb;17(2):410-420. doi: 10.1111/pbi.12986. Epub 2018 Aug 10. Plant Biotechnol J. 2019. PMID: 29993179 Free PMC article.
-
Novel chimeric E2CD154 subunit vaccine is safe and confers long lasting protection against classical swine fever virus.Vet Immunol Immunopathol. 2021 Apr;234:110222. doi: 10.1016/j.vetimm.2021.110222. Epub 2021 Mar 5. Vet Immunol Immunopathol. 2021. PMID: 33690056
-
The challenges of classical swine fever control: modified live and E2 subunit vaccines.Virus Res. 2014 Jan 22;179:1-11. doi: 10.1016/j.virusres.2013.10.025. Epub 2013 Nov 6. Virus Res. 2014. PMID: 24211665 Review.
-
Application of Quillaja saponaria extracts as oral adjuvants for plant-made vaccines.Expert Opin Biol Ther. 2004 Jun;4(6):947-58. doi: 10.1517/14712598.4.6.947. Expert Opin Biol Ther. 2004. PMID: 15174976 Review.
Cited by
-
Enhancement in humoral response against inactivated Newcastle disease vaccine in broiler chickens administered orally with plant-derived soyasaponin.Poult Sci. 2020 Apr;99(4):1921-1927. doi: 10.1016/j.psj.2019.11.050. Epub 2020 Feb 27. Poult Sci. 2020. PMID: 32241472 Free PMC article.
-
The Development of Classical Swine Fever Marker Vaccines in Recent Years.Vaccines (Basel). 2022 Apr 13;10(4):603. doi: 10.3390/vaccines10040603. Vaccines (Basel). 2022. PMID: 35455351 Free PMC article. Review.
-
Circular mRNA-LNP vaccine encoding self-assembled E2-TMD-mi3 nanoparticles licit enhanced CSFV-specific immunity over commercial subunit vaccine.Front Immunol. 2025 Jun 19;16:1604677. doi: 10.3389/fimmu.2025.1604677. eCollection 2025. Front Immunol. 2025. PMID: 40612955 Free PMC article.
-
Research Progress and Challenges in Vaccine Development against Classical Swine Fever Virus.Viruses. 2021 Mar 10;13(3):445. doi: 10.3390/v13030445. Viruses. 2021. PMID: 33801868 Free PMC article. Review.
-
Triterpenoid Saponins from Washnut (Sapindus mukorossi Gaertn.)-A Source of Natural Surfactants and Other Active Components.Plants (Basel). 2022 Sep 9;11(18):2355. doi: 10.3390/plants11182355. Plants (Basel). 2022. PMID: 36145756 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

