Immunogenicity of Protein Pharmaceuticals
- PMID: 30599169
- PMCID: PMC6720129
- DOI: 10.1016/j.xphs.2018.12.014
Immunogenicity of Protein Pharmaceuticals
Abstract
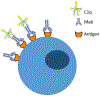
Protein therapeutics have drastically changed the landscape of treatment for many diseases by providing a regimen that is highly specific and lacks many off-target toxicities. The clinical utility of many therapeutic proteins has been undermined by the potential development of unwanted immune responses against the protein, limiting their efficacy and negatively impacting its safety profile. This review attempts to provide an overview of immunogenicity of therapeutic proteins, including immune mechanisms and factors influencing immunogenicity, impact of immunogenicity, preclinical screening methods, and strategies to mitigate immunogenicity.
Keywords: antibody(s); immune response(s); immunogenicity; immunology; pharmacodynamics; pharmacokinetics; protein(s).
Copyright © 2019 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, Mackey J, Kishnani P, Smith W, McVie-Wylie A 2001. Recombinant human acid &agr;-glucosidase enzyme therapy for infantile glycogen storage disease type II: Results of a phase I/II clinical trial. Genetics in Medicine 3(2):132–138. - PubMed
-
- Klinge L, Straub V, Neudorf U, Schaper J, Bosbach T, Görlinger K, Wallot M, Richards S, Voit T 2005. Safety and efficacy of recombinant acid alpha-glucosidase (rhGAA) in patients with classical infantile Pompe disease: results of a phase II clinical trial. Neuromuscular Disorders 15(1):24–31. - PubMed
-
- Lusher JM, Arkin S, Abildgaard CF, Schwartz RS 1993. Recombinant Factor VIII for the Treatment of Previously Untreated Patients with Hemophilia A -- Safety, Efficacy, and Development of Inhibitors. New England Journal of Medicine 328(7):453–459. - PubMed
-
- Rudick RA, Ransohoff RM, Lee JC, Peppler R, Yu M, Mathisen PM, Tuohy VK 1998. In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology 50(5):1294–1300. - PubMed
-
- Herndon RM, Rudick RA, Munschauer FE III, Mass MK, Salazar AM, Coats ME, Labutta R, Richert JR, Cohan SL, Genain C 2005. Eight-year immunogenicity and safety of interferon beta-1a-Avonex® treatment in patients with multiple sclerosis. Multiple Sclerosis Journal 11(4):409–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

