Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non-Small Cell Lung Cancer
- PMID: 30599201
- PMCID: PMC7310567
- DOI: 10.1016/j.jtho.2018.12.014
Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non-Small Cell Lung Cancer
Abstract
Introduction: Clinical variables describing the natural history and longitudinal therapy outcomes of stage IV anaplastic lymphoma kinase gene rearrangement positive (ALK-positive) NSCLC and their relationship with long-term overall survival (OS) have not previously been described in detail.
Methods: Patients with stage IV NSCLC treated with an ALK inhibitor at the University of Colorado Cancer Center from 2009 through November 2017 were identified retrospectively. OS curves were constructed by using Kaplan-Meier methods. Multivariate Cox proportional hazard analysis was used to determine the relationship of variables with OS.
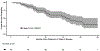
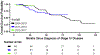
Results: Of the 110 patients with ALK-positive NSCLC who were identified, 105 received crizotinib as their initial ALK inhibitor. With a median follow-up time of 47 months, the median OS time from diagnosis of stage IV disease was 81 months (6.8 years). Brain metastases at diagnosis of stage IV disease (hazard ratio = 1.01, p = 0.971) and year of stage IV presentation (p = 0.887) did not influence OS. More organs with tumor at diagnosis of stage IV disease was associated with worse OS (HR = 1.49 for each additional organ with disease, including the CNS [p = 0.002]). Each additional month of pemetrexed-based therapy was associated with a 7% relative decrease in risk of death.
Conclusion: Patients with stage IV ALK-positive NSCLC can have prolonged OS. Brain metastases at diagnosis of stage IV disease does not influence OS. Having more organs involved with tumor at stage IV presentation is associated with worse outcomes. Prolonged benefit from pemetrexed is associated with better outcomes.
Keywords: ALK; Anaplastic lymphoma kinase; Non–small cell lung cancer; Overall survival; Stage IV.
Copyright © 2018 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure: Dr. Pacheco has received consulting fees from AstraZeneca and Novartis, honoraria from Takeda, and research funding from Pfizer. Dr. Hancock has received speaker fees from Guardant. Dr. Bunn has received consulting fees from AstraZeneca, Roche/Genentech, Pfizer, and Takeda. Dr. Gaspar has received honoraria from AstraZeneca. Dr Rusthoven has received honoraria from Takeda. Dr. Aisner has received consulting fees from AbbVie, Bristol-Myers Squibb and Inivata. Dr. Doebele has received consulting fees from AstraZeneca, Ignyta, and Takeda; research funding from Ignyta, and licensing fees from Abbott Molecular, Ignyta; and Rain Therapeutics, and he owns stock in Rain Therapeutics. Dr. Camidge has received consulting fees from Takeda, Arrys/Kyn, AstraZeneca, Bio-Thera, Celgene, Clovis, Daiichi Sankyo, Genoptix, G1 Therapeutics, Hansoh, Hengrui, Ignyta, Lycera, Mersana Therapeutics, Novartis, Orion, Regeneron, Revolution Medicine, and Roche/Genentech and research funding from Takeda. The remaining authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

