Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation
- PMID: 30600260
- PMCID: PMC7175813
- DOI: 10.1126/scisignal.aar6889
Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation
Abstract
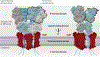
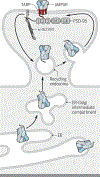
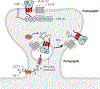
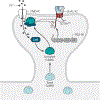
l-Glutamate is the main excitatory neurotransmitter in the brain, with postsynaptic responses to its release predominantly mediated by AMPA-type glutamate receptors (AMPARs). A critical component of synaptic plasticity involves changes in the number of responding postsynaptic receptors, which are dynamically recruited to and anchored at postsynaptic sites. Emerging findings continue to shed new light on molecular mechanisms that mediate AMPAR postsynaptic trafficking and localization. Accordingly, unconventional secretory trafficking of AMPARs occurs in dendrites, from the endoplasmic reticulum (ER) through the ER-Golgi intermediary compartment directly to recycling endosomes, independent of the Golgi apparatus. Upon exocytosis, AMPARs diffuse in the plasma membrane to reach the postsynaptic site, where they are trapped to contribute to transmission. This trapping occurs through a combination of both intracellular interactions, such as TARP (transmembrane AMPAR regulatory protein) binding to α-actinin-stabilized PSD-95, and extracellular interactions through the receptor amino-terminal domain. These anchoring mechanisms may facilitate precise receptor positioning with respect to glutamate release sites to enable efficient synaptic transmission.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

References
-
- Greger IH, Watson JF, Cull-Candy SG, Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 94, 713–730 (2017). - PubMed

