Next-generation sequencing in precision oncology: Patient understanding and expectations
- PMID: 30600607
- PMCID: PMC6346219
- DOI: 10.1002/cam4.1947
Next-generation sequencing in precision oncology: Patient understanding and expectations
Abstract
Background: Implementation of precision oncology interventions poses several challenges to informed consent and patient education. This study assessed cancer patients' understanding, expectations, and outcomes regarding participation in research examining the impact of matched tumor and germline sequencing on their clinical care.
Methods: A total of 297 patients (mean age: 59 years; 50% female; 96% white) with refractory, metastatic cancer were surveyed, including 217 who completed surveys both before and after undergoing integrated whole exome and transcriptome sequencing as part of a larger clinical research study.
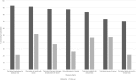
Results: At baseline, the vast majority of patients expected to receive several potential direct benefits from study participation, including written reports of sequencing findings (88%), greater understanding of the causes of their cancer (74%), and participation in clinical trials for which sequencing results would make them eligible (84%). In most cases, these benefits were not realized by study completion. Despite explanations from study personnel to the contrary, most participants (67%-76%) presumed that incidental germline sequencing findings relevant to noncancerous health conditions (eg, diabetes) would automatically be disclosed to them. Patients reported low levels of concern about study risks at baseline and low levels of regret about study participation at follow-up.
Conclusions: Findings suggest that cancer patients participating in precision oncology intervention research have largely unfulfilled expectations of direct benefits related to their study participation. Increased focus on patient education to supplement the informed consent process may help manage patients' expectations regarding the extent and likelihood of benefits received as a result of undergoing genomic sequencing.
Keywords: genome sequencing; informed consent; patient education; precision oncology.
© 2018 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Fiore RN, Goodman KW. Precision medicine ethics: selected issues and developments in next‐generation sequencing, clinical oncology, and ethics. Curr Opin Oncol. 2016;28:83‐87. - PubMed