[Effects of adipose-derived stem cell released exosomes on proliferation, migration, and tube-like differentiation of human umbilical vein endothelial cells]
- PMID: 30600670
- PMCID: PMC8414149
- DOI: 10.7507/1002-1892.201804016
[Effects of adipose-derived stem cell released exosomes on proliferation, migration, and tube-like differentiation of human umbilical vein endothelial cells]
Abstract
Objective: To explore the effects of adipose-derived stem cell released exosomes (ADSC-Exos) on the proliferation, migration, and tube-like differentiation of human umbilical vein endothelial cells (HUVECs).
Methods: Adipose tissue voluntarily donated by liposuction patients was obtained. The ADSCs were harvested by enzyme digestion and identified by flow cytometry and adipogenic induction. The ADSC-Exos were extracted from the supernatant of the 3rd generation ADSCs and the morphology was observed by transmission electron microscopy. The surface proteins (Alix and CD63) were detected by Western blot. The nanoparticle tracking analyzer NanoSight was used to analyze the size distribution of ADSC-Exos. After co-culture of PKH26 fluorescently labeled ADSC-Exos with HUVECs, confocal microscopy had been used to observe whether ADSC-Exos could absorbed by HUVECs. ADSC-Exos and HUVECs were co-cultured for 1, 2, 3, 4, and 5 days. The effect of ADSC-Exos on the proliferation of HUVECs was detected by cell counting kit 8 (CCK-8) assay. The expression of VEGF protein in the supernatant of HUVECs with or without ADSC-Exos had been detected by ELISA after 12 hours. Transwell migration assay was used to detect the effect of ADSC-Exos on the migration ability of HUVECs. The effect of ADSC-Exos on the tubular structure formation of HUVECs was observed by Matrigel experiments in vitro. The formation of subcutaneous tubular structure in vivo was observed in BALB/c male nude mice via the injection of HUVECs and Matrigel with or without ADSC-Exos. After 2 weeks, the neovascularization in Matrigel was measured and mean blood vessel density (MVD) was calculated. The above experiments were all controlled by the same amount of PBS.
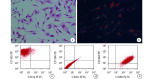
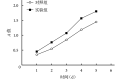
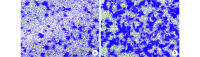
Results: After identification, the cultured cells were consistent with the characteristics of ADSCs. ADSC-Exos were circular or elliptical membranous vesicle with uniform morphology under transmission electron microscopy, and expresses the signature proteins Alix and CD63 with particle size ranging from 30 to 200 nm. Confocal microscopy results showed that ADSC-Exos could be absorbed by HUVECs. The CCK-8 analysis showed that the cell proliferation of the experimental group was better than that of the control group at each time point ( P<0.05). The result of Transwell showed that the trans-membrane migration cells in the experimental group were significantly more than that in the control group ( t=9.534, P=0.000). In vitro, Matrigel tube-forming experiment showed that the number of tube-like structures in the experimental group was significantly higher than that of the control group ( t=15.910, P=0.000). In vivo, the MVD of the experimental group was significantly higher than that of the control group ( t=16.710, P=0.000). The ELISA assay showed that the expression of VEGF protein in the supernatant of the experimental group was significantly higher than that of the control group ( t=21.470, P=0.000).
Conclusion: ADSC-Exos can promote proliferation, migration, and tube-like structure formation of HUVECs, suggesting that ADSC-Exos can promote angiogenesis in vitro and in vivo.
目的: 探讨脂肪干细胞来源外泌体(adipose-derived stem cell released exosomes,ADSC-Exos)对人脐静脉血管内皮细胞(human umbilical vein endothelial cells,HUVECs)增殖、迁移及管样分化的影响。.
方法: 取吸脂患者自愿捐赠的脂肪组织,采用酶消化法分离培养获得 ADSCs,并行流式细胞检测及成脂诱导鉴定。收集第 3 代 ADSCs 上清液提取外泌体,透射电镜观察其形态,Western blot 检测其膜表面标志性蛋白 Alix 和 CD63,用纳米颗粒跟踪分析仪 NanoSight 分析外泌体粒径分布范围。用 PKH26 荧光标记的 ADSC-Exos 与 HUVECs 共培养后,共聚焦显微镜观察 ADSC-Exos 能否进入 HUVECs。将 ADSC-Exos 与 HUVECs 共培养 1、2、3、4、5 d,CCK-8 法检测 ADSC-Exos 对 HUVECs 增殖影响;共培养 12 h 时 ELISA 法检测细胞上清液 VEGF 蛋白表达量。采用 Transwell 迁移实验检测 ADSC-Exos 对 HUVECs 迁移能力的影响。通过体外 Matrigel 基质胶实验,观察 ADSC-Exos 对 HUVECs 管状结构形成的影响;将 HUVECs 与 Matrigel 基质胶混合后联合 ADSC-Exos 注射在 BALB/c 雄性裸鼠皮下,2 周后检测基质胶中新生血管情况,计算平均血管密度(mean blood vessel density,MVD)。上述实验均以等量 PBS 作为对照。.
结果: 经鉴定所培养细胞符合 ADSCs 的特征。ADSC-Exos 为形态一致的圆形或椭圆形膜性囊泡,表达标志性蛋白 Alix 和 CD63,粒径范围 30~200 nm。共聚焦显微镜观察示 ADSC-Exos 可被 HUVECs 摄取。CCK-8 法检测示,各时间点实验组细胞增殖均优于对照组( P<0.05)。Transwell 实验结果显示,实验组跨膜迁移细胞数较对照组显著增多( t=9.534, P=0.000)。在体外 Matrigel 胶成管实验中,实验组管样结构数明显多于对照组( t=15.910, P=0.000);在体内实验中,实验组 MVD 亦显著高于对照组( t=16.710, P=0.000)。ELISA 检测示,实验组细胞上清液 VEGF 蛋白表达量明显高于对照组( t=21.470, P=0.000)。.
结论: ADSC-Exos 可促进 HUVECs 增殖、迁移及管样结构形成,提示 ADSC-Exos 可促进血管新生。.
Keywords: Adipose-derived stem cells; angiogenesis; exosomes; human umbilical vein endothelial cells.
Figures




Similar articles
-
[Effect of adipose-derived stem cell derived exosomes on angiogenesis after skin flap transplantation in rats].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019 Dec 15;33(12):1560-1565. doi: 10.7507/1002-1892.201904023. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019. PMID: 31823559 Free PMC article. Chinese.
-
[Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020 Jan 15;34(1):124-131. doi: 10.7507/1002-1892.201903058. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020. PMID: 31939247 Free PMC article. Chinese.
-
Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting.Biochem Biophys Res Commun. 2018 Feb 26;497(1):305-312. doi: 10.1016/j.bbrc.2018.02.076. Epub 2018 Feb 8. Biochem Biophys Res Commun. 2018. PMID: 29428734
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Clinical Applications of Adipose-Derived Stem Cell (ADSC) Exosomes in Tissue Regeneration.Int J Mol Sci. 2024 May 29;25(11):5916. doi: 10.3390/ijms25115916. Int J Mol Sci. 2024. PMID: 38892103 Free PMC article. Review.
Cited by
-
[Effect of adipose-derived stem cell derived exosomes on angiogenesis after skin flap transplantation in rats].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019 Dec 15;33(12):1560-1565. doi: 10.7507/1002-1892.201904023. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019. PMID: 31823559 Free PMC article. Chinese.
-
[Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020 Jan 15;34(1):124-131. doi: 10.7507/1002-1892.201903058. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020. PMID: 31939247 Free PMC article. Chinese.
-
Exosomes and Signaling Nanovesicles from the Nanofiltration of Preconditioned Adipose Tissue with Skin-B® in Tissue Regeneration and Antiaging: A Clinical Study and Case Report.Medicina (Kaunas). 2024 Apr 21;60(4):670. doi: 10.3390/medicina60040670. Medicina (Kaunas). 2024. PMID: 38674316 Free PMC article. Clinical Trial.
-
[Effect of exosomes from adipose-derived stem cells on peripheral nerve regeneration].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Dec 15;32(12):1592-1596. doi: 10.7507/1002-1892.201707051. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 30569689 Free PMC article. Chinese.
-
[Advance of vascularization of tissue engineered peripheral nerve].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019 Aug 15;33(8):1029-1032. doi: 10.7507/1002-1892.201902032. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019. PMID: 31407564 Free PMC article. Chinese.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
