Palladium/Norbornene-Catalyzed Indenone Synthesis from Simple Aryl Iodides: Concise Syntheses of Pauciflorol F and Acredinone A
- PMID: 30600880
- PMCID: PMC6420343
- DOI: 10.1002/anie.201813699
Palladium/Norbornene-Catalyzed Indenone Synthesis from Simple Aryl Iodides: Concise Syntheses of Pauciflorol F and Acredinone A
Abstract
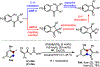
To show the synthetic utility of palladium/norbornene (Pd/NBE) cooperative catalysis, here we report concise syntheses of indenone-based natural products, pauciflorol F and acredinone A, which are enabled by direct annulation between aryl iodides and unsaturated carboxylic acid anhydrides. Compared to the previous indenone-preparation approaches, this method allows simple aryl iodides to be used as substrates with complete control of the regioselectivity. The total synthesis of acredinone A features two different Pd/NBE-catalyzed ortho acylation reactions for constructing penta-substituted arene cores, including the development of a new ortho acylation/ipso borylation.
Keywords: acredinone A; indenone; norbornene; palladium; pauciflorol F.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Modular ipso/ ortho Difunctionalization of Aryl Bromides via Palladium/Norbornene Cooperative Catalysis.J Am Chem Soc. 2018 Jul 11;140(27):8551-8562. doi: 10.1021/jacs.8b04153. Epub 2018 Jun 27. J Am Chem Soc. 2018. PMID: 29906109 Free PMC article.
-
Ortho C-H Acylation of Aryl Iodides by Palladium/Norbornene Catalysis.Angew Chem Int Ed Engl. 2015 Oct 19;54(43):12664-8. doi: 10.1002/anie.201506397. Epub 2015 Sep 1. Angew Chem Int Ed Engl. 2015. PMID: 26333071
-
Palladium/norbornene-catalyzed synthesis of heteroatom-containing o-teraryls from aryl iodides and heteroarenes through double C-H activation in sequence.Chemistry. 2009 Aug 10;15(32):7850-7853. doi: 10.1002/chem.200900843. Chemistry. 2009. PMID: 19466733 No abstract available.
-
Structurally Modified Norbornenes: A Key Factor to Modulate Reaction Selectivity in the Palladium/Norbornene Cooperative Catalysis.J Am Chem Soc. 2020 Oct 21;142(42):17859-17875. doi: 10.1021/jacs.0c09193. Epub 2020 Oct 5. J Am Chem Soc. 2020. PMID: 33016072 Free PMC article. Review.
-
Palladium/Norbornene Cooperative Catalysis.Chem Rev. 2019 Jun 26;119(12):7478-7528. doi: 10.1021/acs.chemrev.9b00079. Epub 2019 Apr 25. Chem Rev. 2019. PMID: 31021606 Free PMC article. Review.
Cited by
-
Escape from Palladium: Nickel-Catalyzed Catellani Annulation.J Am Chem Soc. 2023 May 24;145(20):11005-11011. doi: 10.1021/jacs.3c03780. Epub 2023 May 15. J Am Chem Soc. 2023. PMID: 37184338 Free PMC article.
-
Concise Total Syntheses of Leuconoxine-Type Alkaloids Enabled by Palladium/Norbornene-Catalyzed Pyrrole Difunctionalization.Angew Chem Int Ed Engl. 2025 Jun 2;64(23):e202502736. doi: 10.1002/anie.202502736. Epub 2025 Apr 10. Angew Chem Int Ed Engl. 2025. PMID: 40163013 Free PMC article.
-
Sulfenamide-enabled ortho thiolation of aryl iodides via palladium/norbornene cooperative catalysis.Nat Commun. 2019 Aug 7;10(1):3555. doi: 10.1038/s41467-019-11398-0. Nat Commun. 2019. PMID: 31391472 Free PMC article.
-
Rapid Access to Multisubstituted Acrylamides from Cyclic Ketones via Palladium/Norbornene Cooperative Catalysis.Angew Chem Int Ed Engl. 2022 Apr 19;61(17):e202201239. doi: 10.1002/anie.202201239. Epub 2022 Mar 4. Angew Chem Int Ed Engl. 2022. PMID: 35199445 Free PMC article.
-
Asymmetric total synthesis of benzenoid cephalotane-type diterpenoids through a cascade C(sp2) & C(sp3)-H activation.Nat Commun. 2025 May 20;16(1):4674. doi: 10.1038/s41467-025-59816-w. Nat Commun. 2025. PMID: 40393997 Free PMC article.
References
-
- Marvel CS, Hinman CW, J. Am. Chem. Soc 1954, 76, 5435;
- Martens H, Hoornaer G, Tetrahedron 1974, 30, 3641;
- Vasilyev AV, Walspurger S, Haouas M, Sommer J, Pale P, Rudenko AP, Org. Biomol. Chem 2004, 2, 3483; - PubMed
- Vasilyev AV, Walspurger S, Pale P, Sommer J, Tetrahedron Lett 2004, 45, 3379;
- Pan C, Huang B, Hu W, Feng X, Yu J, J. Org. Chem 2016, 81, 2087; - PubMed
- Zhang X-S, Jiao J-Y, Zhang X-H, Hu B-L, Zhang X-G, J. Org. Chem 2016, 81, 5710; - PubMed
- Suchand B, Satyanarayana G, J. Org. Chem 2017, 82, 372. - PubMed
-
- Larock RC, Doty MJ, Cacchi S, J. Org. Chem 1993, 58, 4579;
- Alonso DA, Najera C, Pacheco MC, Adv. Synth. Catal 2002, 344, 172;
- Pletnev AA, Tian Q, Larock RC, J. Org. Chem 2002, 67, 9276; - PubMed
- Miura T, Murakami M, Org. Lett 2005, 7, 3339; - PubMed
- Harada Y, Nakanishi J, Fujihara H, Tobisu M, Fukumoto Y, Chatani N, J. Am. Chem. Soc 2007, 129, 5766; - PubMed
- Tsukamoto H, Kondo Y, Org. Lett 2007, 9, 4227; - PubMed
- Liu CC, Korivi RP, Cheng CH, Chem. Eur. J 2008, 14, 9503; - PubMed
- Morimoto T, Yamasaki K, Hirano A, Tsutsumi K, Kagawa N, Kakiuchi K, Harada Y, Fukumoto Y, Chatani N, Nishioka T, Org. Lett 2009, 11, 1777; - PubMed
- Yan X, Zou S, Zhao P, Xi C, Chem. Commun 2014, 50, 2775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources