Fluence rate dependence of red light-induced phosphorylation of plasma membrane H+-ATPase in stomatal guard cells
- PMID: 30601076
- PMCID: PMC6351090
- DOI: 10.1080/15592324.2018.1561107
Fluence rate dependence of red light-induced phosphorylation of plasma membrane H+-ATPase in stomatal guard cells
Abstract
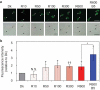
Stomatal opening is induced by red light as well as blue light. Recently, we established an immunohistochemical technique using whole leaves to study plasma membrane (PM) H+-ATPase in guard cells, which is an important enzyme driving stomatal opening. Our technique revealed that red light illuminated to whole leaves induces photosynthesis-dependent phosphorylation of C-terminal penultimate residue of PM H+-ATPase, threonine, in guard cells, which has been considered to be important for activation of PM H+-ATPase, and we proposed that red light promotes stomatal opening via activation of PM H+-ATPase in guard cells in whole leaves. Here, using our new immunohistochemical technique, we investigated fluence rate dependence of red light-induced phosphorylation of PM H+-ATPase. We found that illumination of red light at 50 µmol m-2 s-1, which was suggested to initiate photosynthesis, saturates phosphorylation of PM H+-ATPase. Furthermore, we immunohistochemically confirmed decrease in the amount of PM H+-ATPase protein in a knock-out mutant of AHA1, an isogene encoding the major isoform of PM H+-ATPase in guard cells, implying the importance of AHA1 as the major PM H+-ATPase protein in guard cells for light-induced stomatal opening.
Keywords: guard cells; immunohistochemistry; phosphorylation; photosynthesis; plasma membrane H-ATPase; red light.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
