Functional significance of the platelet immune receptors GPVI and CLEC-2
- PMID: 30601137
- PMCID: PMC6307936
- DOI: 10.1172/JCI122955
Functional significance of the platelet immune receptors GPVI and CLEC-2
Abstract
Although platelets are best known for their role in hemostasis, they are also crucial in development, host defense, inflammation, and tissue repair. Many of these roles are regulated by the immune-like receptors glycoprotein VI (GPVI) and C-type lectin receptor 2 (CLEC-2), which signal through an immunoreceptor tyrosine-based activation motif (ITAM). GPVI is activated by collagen in the subendothelial matrix, by fibrin and fibrinogen in the thrombus, and by a remarkable number of other ligands. CLEC-2 is activated by the transmembrane protein podoplanin, which is found outside of the vasculature and is upregulated in development, inflammation, and cancer, but there is also evidence for additional ligands. In this Review, we discuss the physiological and pathological roles of CLEC-2 and GPVI and their potential as targets in thrombosis and thrombo-inflammatory disorders (i.e., disorders in which inflammation plays a critical role in the ensuing thrombosis) relative to current antiplatelet drugs.
Conflict of interest statement
Figures
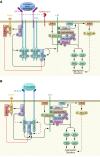


References
-
- Miura Y, Takahashi T, Jung SM, Moroi M. Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J Biol Chem. 2002;277(48):46197–46204. doi: 10.1074/jbc.M204029200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

