Nitric Oxide Donor Prevents Neonatal Isoflurane-induced Impairments in Synaptic Plasticity and Memory
- PMID: 30601214
- PMCID: PMC6538043
- DOI: 10.1097/ALN.0000000000002529
Nitric Oxide Donor Prevents Neonatal Isoflurane-induced Impairments in Synaptic Plasticity and Memory
Abstract
What we already know about this topic: Some general anesthetics have been shown to have adverse effects on neuronal development that affect neural function and cognitive behavior.Clinically relevant concentrations of inhalational anesthetics inhibit the postsynaptic density (PSD)-95, discs large homolog, and zona occludens-1 (PDZ) domain-mediated protein-protein interaction between PSD-95 or PSD-93 and N-methyl-D-aspartate receptors or neuronal NO synthase.
What this article tells us that is new: Neonatal PSD-95 PDZ2WT peptide treatment mimics the effects of isoflurane (~1 minimum alveolar concentration) by altering dendritic spine morphology, neural plasticity, and memory without inducing detectable increases in apoptosis or changes in synaptic density.These results indicate that a single dose of isoflurane (~1 minimum alveolar concentration) or PSD-95 PDZ2WT peptide alters dendritic spine architecture and functions important for cognition in the developing brain. This impairment can be prevented by administration of the NO donor molsidomine.
Background: In humans, multiple early exposures to procedures requiring anesthesia constitute a significant risk factor for development of learning disabilities and disorders of attention. In animal studies, newborns exposed to anesthetics develop long-term deficits in cognition. Previously, our laboratory showed that postsynaptic density (PSD)-95, discs large homolog, and zona occludens-1 (PDZ) domains may serve as a molecular target for inhaled anesthetics. This study investigated a role for PDZ interactions in spine development, plasticity, and memory as a potential mechanism for early anesthetic exposure-produced cognitive impairment.
Methods: Postnatal day 7 mice were exposed to 1.5% isoflurane for 4 h or injected with 8 mg/kg active PSD-95 PDZ2WT peptide. Apoptosis, hippocampal dendritic spine changes, synapse density, long-term potentiation, and cognition functions were evaluated (n = 4 to 18).
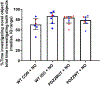
Results: Exposure of postnatal day 7 mice to isoflurane or PSD-95 PDZ2WT peptide causes a reduction in long thin spines (median, interquartile range [IQR]: wild type control [0.54, 0.52 to 0.86] vs. wild type isoflurane [0.31, 0.16 to 0.38], P = 0.034 and PDZ2MUT [0.86, 0.67 to 1.0] vs. PDZ2WT [0.55, 0.53 to 0.59], P = 0.028), impairment in long-term potentiation (median, IQR: wild type control [123, 119 to 147] and wild type isoflurane [101, 96 to 118], P = 0.049 and PDZ2MUT [125, 119 to 131] and PDZ2WT [104, 97 to 107], P = 0.029), and deficits in acute object recognition (median, IQR: wild type control [79, 72 to 88] vs. wild type isoflurane [63, 55 to 72], P = 0.044 and PDZ2MUT [81, 69 to 84] vs. PDZ2WT [67, 57 to 77], P = 0.039) at postnatal day 21 without inducing detectable differences in apoptosis or changes in synaptic density. Impairments in recognition memory and long-term potentiation were preventable by introduction of a NO donor.
Conclusions: Early disruption of PDZ domain-mediated protein-protein interactions alters spine morphology, synaptic function, and memory. These results support a role for PDZ interactions in early anesthetic exposure-produced cognitive impairment. Prevention of recognition memory and long-term potentiation deficits with a NO donor supports a role for the N-methyl-D-aspartate receptor/PSD-95/neuronal NO synthase pathway in mediating these aspects of isoflurane-induced cognitive impairment.
Conflict of interest statement
Figures





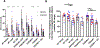

Similar articles
-
Disruption of Extracellular Signal-Regulated Kinase Partially Mediates Neonatal Isoflurane Anesthesia-Induced Changes in Dendritic Spines and Cognitive Function in Juvenile Mice.Int J Mol Sci. 2025 Jan 24;26(3):981. doi: 10.3390/ijms26030981. Int J Mol Sci. 2025. PMID: 39940749 Free PMC article.
-
Neonatal Isoflurane Anesthesia or Disruption of Postsynaptic Density-95 Protein Interactions Change Dendritic Spine Densities and Cognitive Function in Juvenile Mice.Anesthesiology. 2020 Oct 1;133(4):812-823. doi: 10.1097/ALN.0000000000003482. Anesthesiology. 2020. PMID: 32773681 Free PMC article.
-
Isoflurane Disrupts Postsynaptic Density-95 Protein Interactions Causing Neuronal Synapse Loss and Cognitive Impairment in Juvenile Mice via Canonical NO-mediated Protein Kinase-G Signaling.Anesthesiology. 2022 Aug 1;137(2):212-231. doi: 10.1097/ALN.0000000000004264. Anesthesiology. 2022. PMID: 35504002 Free PMC article.
-
[Volatile anesthetics and synaptic transmission in the central nervous system].Masui. 2004 Aug;53(8):873-81. Masui. 2004. PMID: 15446675 Review. Japanese.
-
PDZ Domains as Drug Targets.Adv Ther (Weinh). 2019 Jul;2(7):1800143. doi: 10.1002/adtp.201800143. Epub 2019 Apr 24. Adv Ther (Weinh). 2019. PMID: 32313833 Free PMC article. Review.
Cited by
-
Disruption of Extracellular Signal-Regulated Kinase Partially Mediates Neonatal Isoflurane Anesthesia-Induced Changes in Dendritic Spines and Cognitive Function in Juvenile Mice.Int J Mol Sci. 2025 Jan 24;26(3):981. doi: 10.3390/ijms26030981. Int J Mol Sci. 2025. PMID: 39940749 Free PMC article.
-
Neuroplastic Changes in the Superior Colliculus and Hippocampus in Self-rewarding Paradigm: Importance of Visual Cues.Mol Neurobiol. 2022 Feb;59(2):890-915. doi: 10.1007/s12035-021-02597-2. Epub 2021 Nov 19. Mol Neurobiol. 2022. PMID: 34797522
-
Dendritic spine remodeling and plasticity under general anesthesia.Brain Struct Funct. 2021 Sep;226(7):2001-2017. doi: 10.1007/s00429-021-02308-6. Epub 2021 Jun 1. Brain Struct Funct. 2021. PMID: 34061250 Free PMC article. Review.
-
Intranasal levosimendan prevents cognitive dysfunction and apoptotic response induced by repeated isoflurane exposure in newborn rats.Naunyn Schmiedebergs Arch Pharmacol. 2021 Jul;394(7):1553-1567. doi: 10.1007/s00210-021-02077-3. Epub 2021 Mar 27. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33772342
-
From neurotoxicity to neuroprotection: Rethinking GABAAR-targeting anesthetics.Cell Biol Toxicol. 2025 Jun 14;41(1):104. doi: 10.1007/s10565-025-10057-z. Cell Biol Toxicol. 2025. PMID: 40516005 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

