In vitro and in vivo mouse follicle development in ovaries and reaggregated ovaries
- PMID: 30601757
- PMCID: PMC6347279
- DOI: 10.1530/REP-18-0115
In vitro and in vivo mouse follicle development in ovaries and reaggregated ovaries
Abstract
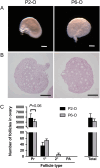
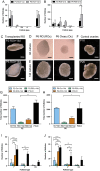
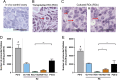
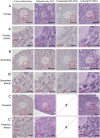
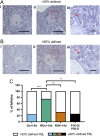
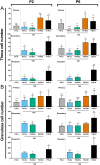
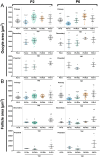
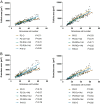
Follicle development requires complex and coordinated interactions between both the oocyte and its associated somatic cells. In ovarian dysfunction, follicle development may be abnormal due to defective somatic cell function; for example, premature ovarian insufficiency or malignancies. Replacing defective somatic cells, using the reaggregated ovary (RO) technique, may 'rescue' follicle development. ROs containing mature follicles have been generated when transplanted to a host mouse to develop. We have developed a RO culture technique and the aims were to determine how follicle development differed between transplanted and cultured ROs, and the influence of ovarian age (P2 vs P6). Mouse ROs were cultured for 14 days; P2 and P6 ovaries cultured as Controls. Follicle development was compared to ROs transplanted for 14 days and ovaries from P16 and P20 mice. ROs generated from either P2 or P6 exhibited similar follicle development in culture whereas in vivo follicle development was more advanced in P6 ROs. Follicles were more developed in cultured ROs than transplanted ROs. However, follicles in cultured ROs and ovaries had smaller oocytes with fewer theca and granulosa cells than in vivo counterparts. Our results demonstrate the fluidity of follicle development despite ovary dissociation and that environment is more important to basal lamina formation and theca cell development. Furthermore, follicle development within cultured ROs appears to be independent of oocyte nest breakdown and primordial follicle formation in source ovaries. Our results highlight the need for understanding follicle development in vitro, particularly in the development of the RO technique as a potential fertility treatment.
Conflict of interest statement
Suzannah Williams is a member of the Editorial Board of Reproduction. The other authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

