Conserved function of the matriptase-prostasin proteolytic cascade during epithelial morphogenesis
- PMID: 30601807
- PMCID: PMC6331135
- DOI: 10.1371/journal.pgen.1007882
Conserved function of the matriptase-prostasin proteolytic cascade during epithelial morphogenesis
Abstract
Extracellular matrix (ECM) assembly and remodelling is critical during development and organ morphogenesis. Dysregulation of ECM is implicated in many pathogenic conditions, including cancer. The type II transmembrane serine protease matriptase and the serine protease prostasin are key factors in a proteolytic cascade that regulates epithelial ECM differentiation during development in vertebrates. Here, we show by rescue experiments that the Drosophila proteases Notopleural (Np) and Tracheal-prostasin (Tpr) are functional homologues of matriptase and prostasin, respectively. Np mediates morphogenesis and remodelling of apical ECM during tracheal system development and is essential for maintenance of the transepithelial barrier function. Both Np and Tpr degrade the zona pellucida-domain (ZP-domain) protein Dumpy, a component of the transient tracheal apical ECM. Furthermore, we demonstrate that Tpr zymogen and the ZP domain of the ECM protein Piopio are cleaved by Np and matriptase in vitro. Our data indicate that the evolutionarily conserved ZP domain, present in many ECM proteins of vertebrates and invertebrates, is a novel target of the conserved matriptase-prostasin proteolytic cascade.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

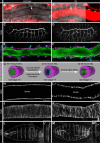
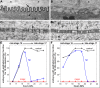

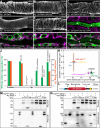

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

