Generating Vegfr3 reporter transgenic mouse expressing membrane-tagged Venus for visualization of VEGFR3 expression in vascular and lymphatic endothelial cells
- PMID: 30601868
- PMCID: PMC6314617
- DOI: 10.1371/journal.pone.0210060
Generating Vegfr3 reporter transgenic mouse expressing membrane-tagged Venus for visualization of VEGFR3 expression in vascular and lymphatic endothelial cells
Abstract
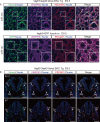
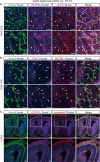
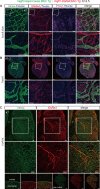
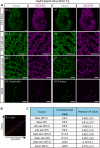
Vascular endothelial growth factor receptor 3 (Vegfr3) has been widely used as a marker for lymphatic and vascular endothelial cells during mouse embryonic development and in adult mouse, making it valuable for studying angiogenesis and lymphangiogenesis under normal and pathological conditions. Here, we report the generation of a novel transgenic (Tg) mouse that expresses a membrane-localized fluorescent reporter protein, Gap43-Venus, under the control of the Vegfr3 regulatory sequence. Vegfr3-Gap43-Venus BAC Tg recapitulated endogenous Vegfr3 expression in vascular and lymphatic endothelial cells during embryonic development and tumor development. Thus, this Tg mouse line contributes a valuable model to study angiogenesis and lymphangiogenesis in physiological and pathological contexts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92: 3566–70. Available: http://www.ncbi.nlm.nih.gov/pubmed/7724599 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

