A microbial-based cancer vaccine for induction of EGFRvIII-specific CD8+ T cells and anti-tumor immunity
- PMID: 30601871
- PMCID: PMC6314576
- DOI: 10.1371/journal.pone.0209153
A microbial-based cancer vaccine for induction of EGFRvIII-specific CD8+ T cells and anti-tumor immunity
Abstract
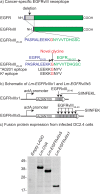
Dysregulated signaling via the epidermal growth factor receptor (EGFR)-family is believed to contribute to the progression of a diverse array of cancers. The most common variant of EGFR is EGFRvIII, which results from a consistent and tumor-specific in-frame deletion of exons 2-7 of the EGFR gene. This deletion generates a novel glycine at the junction and leads to constitutive ligand-independent activity. This junction forms a novel shared tumor neo-antigen with demonstrated immunogenicity in both mice and humans. A 21-amino acid peptide spanning the junctional region was selected, and then one or five copies of this 21-AA neo-peptide were incorporated into live-attenuated Listeria monocytogenes-based vaccine vector. These vaccine candidates demonstrated efficient secretion of the recombinant protein and potent induction of EGFRvIII-specific CD8+ T cells, which prevented growth of an EGFRvIII-expressing squamous cell carcinoma. These data demonstrate the potency of a novel cancer-specific vaccine candidate that can elicit EGFRvIII-specific cellular immunity, for the purpose of targeting EGFRvIII positive cancers that are resistant to conventional therapies.
Conflict of interest statement
P. Lauer and B. Hanson have ownership interest (including patents) in Aduro Biotech. Patents relevant to this manuscript held by authors include Patent# US20070207170A1 (Lauer), US9161974B2 (Bahjat), WO2007022511A2 (Bahjat), WO2012068360A1 (Lauer, Bahjat), US10105427B2 (Lauer, Hanson). This does not alter our adherence to PLOS ONE policies on sharing data and materials. All other authors declare that no competing interests exist.
Figures
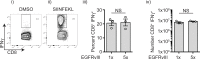



References
-
- Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(2):639–44. Epub 2003/01/08. 10.1073/pnas.232686499 - DOI - PMC - PubMed
-
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313(5998):144–7. Epub 1985/01/10. . - PubMed
-
- Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(19):6899–903. Epub 1987/10/01. - PMC - PubMed
-
- Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(21):8602–6. Epub 1990/11/01. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

