Flexizyme-Enabled Benchtop Biosynthesis of Thiopeptides
- PMID: 30602112
- PMCID: PMC6642631
- DOI: 10.1021/jacs.8b11521
Flexizyme-Enabled Benchtop Biosynthesis of Thiopeptides
Abstract
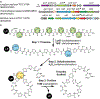
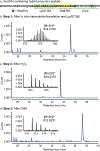
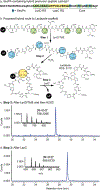
Thiopeptides are natural antibiotics that are fashioned from short peptides by multiple layers of post-translational modification. Their biosynthesis, in particular the pyridine synthases that form the macrocyclic antibiotic core, has attracted intensive research but is complicated by the challenges of reconstituting multiple-pathway enzymes. By combining select RiPP enzymes with cell free expression and flexizyme-based codon reprogramming, we have developed a benchtop biosynthesis of thiopeptide scaffolds. This strategy side-steps several challenges related to the investigation of thiopeptide enzymes and allows access to analytical quantities of new thiopeptide analogs. We further demonstrate that this strategy can be used to validate the activity of new pyridine synthases without the need to reconstitute the cognate prior pathway enzymes.
Figures
Similar articles
-
Structural insights into enzymatic [4+2] aza-cycloaddition in thiopeptide antibiotic biosynthesis.Proc Natl Acad Sci U S A. 2017 Dec 5;114(49):12928-12933. doi: 10.1073/pnas.1716035114. Epub 2017 Nov 20. Proc Natl Acad Sci U S A. 2017. PMID: 29158402 Free PMC article.
-
Total synthesis of thiopeptide antibiotics GE2270A, GE2270T, and GE2270C1.Chem Asian J. 2008 Feb 1;3(2):413-29. doi: 10.1002/asia.200700361. Chem Asian J. 2008. PMID: 18188863
-
Stereoselective synthesis of the gamma-lactam hydrolysate of the thiopeptide cyclothiazomycin.Org Lett. 2004 Sep 16;6(19):3401-4. doi: 10.1021/ol0485870. Org Lett. 2004. PMID: 15355062
-
Introduction to Thiopeptides: Biological Activity, Biosynthesis, and Strategies for Functional Reprogramming.Cell Chem Biol. 2020 Aug 20;27(8):1032-1051. doi: 10.1016/j.chembiol.2020.07.003. Epub 2020 Jul 21. Cell Chem Biol. 2020. PMID: 32698017 Review.
-
From amino acids to heteroaromatics--thiopeptide antibiotics, nature's heterocyclic peptides.Angew Chem Int Ed Engl. 2007;46(42):7930-54. doi: 10.1002/anie.200700728. Angew Chem Int Ed Engl. 2007. PMID: 17854013 Review.
Cited by
-
Substrate Recognition by the Peptidyl-(S)-2-mercaptoglycine Synthase TglHI during 3-Thiaglutamate Biosynthesis.ACS Chem Biol. 2022 Apr 15;17(4):930-940. doi: 10.1021/acschembio.2c00087. Epub 2022 Apr 1. ACS Chem Biol. 2022. PMID: 35362960 Free PMC article.
-
Engineering lanthipeptides by introducing a large variety of RiPP modifications to obtain new-to-nature bioactive peptides.FEMS Microbiol Rev. 2023 May 19;47(3):fuad017. doi: 10.1093/femsre/fuad017. FEMS Microbiol Rev. 2023. PMID: 37096385 Free PMC article. Review.
-
A Compact Reprogrammed Genetic Code for De Novo Discovery of Proteolytically Stable Thiopeptides.J Am Chem Soc. 2024 Mar 27;146(12):8058-8070. doi: 10.1021/jacs.3c12037. Epub 2024 Mar 16. J Am Chem Soc. 2024. PMID: 38491946 Free PMC article.
-
Crossroads of Antibiotic Resistance and Biosynthesis.J Mol Biol. 2019 Aug 23;431(18):3370-3399. doi: 10.1016/j.jmb.2019.06.033. Epub 2019 Jul 6. J Mol Biol. 2019. PMID: 31288031 Free PMC article. Review.
-
Characterization of a Dual Function Peptide Cyclase in Graspetide Biosynthesis.ACS Chem Biol. 2024 Dec 20;19(12):2525-2534. doi: 10.1021/acschembio.4c00626. Epub 2024 Dec 4. ACS Chem Biol. 2024. PMID: 39630567 Free PMC article.
References
-
- Arnison PG; Bibb MJ; Bierbaum G; Bowers AA; Bugni TS; Bulaj G; Camarero JA; Campopiano DJ; Challis GL; Clardy J; et al. Ribosomally Synthesized and Post-Translationally Modified Peptide Natural Products: Overview and Recommendations for a Universal Nomenclature. Nat. Prod. Rep 2012, 30, 108. - PMC - PubMed
-
- van Heel AJ; Mu D; Montalban-Lopez M; Hendriks D; Kuipers OP Designing and Producing Modified, New-to-Nature Peptides with Antimicrobial Activity by Use of a Combination of Various Lantibiotic Modification Enzymes. ACS Synth. Biol 2013, 2, 397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

